Ebola virus protein VP40 stimulates IL-12- and IL-18-dependent activation of human natural killer cells
- PMID: 35862204
- PMCID: PMC9462474
- DOI: 10.1172/jci.insight.158902
Ebola virus protein VP40 stimulates IL-12- and IL-18-dependent activation of human natural killer cells
Abstract
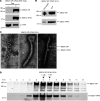
Accumulation of activated natural killer (NK) cells in tissues during Ebola virus infection contributes to Ebola virus disease (EVD) pathogenesis. Yet, immunization with Ebola virus-like particles (VLPs) comprising glycoprotein and matrix protein VP40 provides rapid, NK cell-mediated protection against Ebola challenge. We used Ebola VLPs as the viral surrogates to elucidate the molecular mechanism by which Ebola virus triggers heightened NK cell activity. Incubation of human peripheral blood mononuclear cells with Ebola VLPs or VP40 protein led to increased expression of IFN-γ, TNF-α, granzyme B, and perforin by CD3-CD56+ NK cells, along with increases in degranulation and cytotoxic activity of these cells. Optimal activation required accessory cells like CD14+ myeloid and CD14- cells and triggered increased secretion of numerous inflammatory cytokines. VP40-induced IFN-γ and TNF-α secretion by NK cells was dependent on IL-12 and IL-18 and suppressed by IL-10. In contrast, their increased degranulation was dependent on IL-12 with little influence of IL-18 or IL-10. These results demonstrate that Ebola VP40 stimulates NK cell functions in an IL-12- and IL-18-dependent manner that involves CD14+ and CD14- accessory cells. These potentially novel findings may help in designing improved intervention strategies required to control viral transmission during Ebola outbreaks.
Keywords: Immunology; Infectious disease; Innate immunity; NK cells.
Conflict of interest statement
Figures


Similar articles
-
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins.J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
Role of natural killer cells in innate protection against lethal ebola virus infection.J Exp Med. 2004 Jul 19;200(2):169-79. doi: 10.1084/jem.20032141. Epub 2004 Jul 12. J Exp Med. 2004. PMID: 15249592 Free PMC article.
-
Ebola virus glycoprotein stimulates IL-18-dependent natural killer cell responses.J Clin Invest. 2020 Jul 1;130(7):3936-3946. doi: 10.1172/JCI132438. J Clin Invest. 2020. PMID: 32315287 Free PMC article. Clinical Trial.
-
Cytotoxic Function and Cytokine Production of Natural Killer Cells and Natural Killer T-Like Cells in Systemic Lupus Erythematosis Regulation with Interleukin-15.Mediators Inflamm. 2019 Mar 31;2019:4236562. doi: 10.1155/2019/4236562. eCollection 2019. Mediators Inflamm. 2019. PMID: 31049024 Free PMC article.
-
The Role of Exosomal VP40 in Ebola Virus Disease.DNA Cell Biol. 2017 Apr;36(4):243-248. doi: 10.1089/dna.2017.3639. Epub 2017 Feb 8. DNA Cell Biol. 2017. PMID: 28177658 Free PMC article. Review.
Cited by
-
HIV-1 infection of macrophages differentially primes NK-cell cytotoxicity and proinflammatory cytokine production.iScience. 2025 Jun 11;28(7):112879. doi: 10.1016/j.isci.2025.112879. eCollection 2025 Jul 18. iScience. 2025. PMID: 40678535 Free PMC article.
-
Natural killer cells and their exosomes in viral infections and related therapeutic approaches: where are we?Cell Commun Signal. 2023 Sep 25;21(1):261. doi: 10.1186/s12964-023-01266-2. Cell Commun Signal. 2023. PMID: 37749597 Free PMC article. Review.
-
Animal Model Alternatives in Filovirus and Bornavirus Research.Viruses. 2023 Jan 4;15(1):158. doi: 10.3390/v15010158. Viruses. 2023. PMID: 36680198 Free PMC article. Review.
-
Managing the immune microenvironment of osteosarcoma: the outlook for osteosarcoma treatment.Bone Res. 2023 Feb 27;11(1):11. doi: 10.1038/s41413-023-00246-z. Bone Res. 2023. PMID: 36849442 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

