Influenza Activity and Composition of the 2022-23 Influenza Vaccine - United States, 2021-22 Season
- PMID: 35862284
- PMCID: PMC9310632
- DOI: 10.15585/mmwr.mm7129a1
Influenza Activity and Composition of the 2022-23 Influenza Vaccine - United States, 2021-22 Season
Abstract
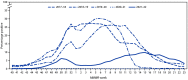
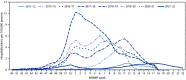
Before the emergence of SARS-CoV-2, the virus that causes COVID-19, influenza activity in the United States typically began to increase in the fall and peaked in February. During the 2021-22 season, influenza activity began to increase in November and remained elevated until mid-June, featuring two distinct waves, with A(H3N2) viruses predominating for the entire season. This report summarizes influenza activity during October 3, 2021-June 11, 2022, in the United States and describes the composition of the Northern Hemisphere 2022-23 influenza vaccine. Although influenza activity is decreasing and circulation during summer is typically low, remaining vigilant for influenza infections, performing testing for seasonal influenza viruses, and monitoring for novel influenza A virus infections are important. An outbreak of highly pathogenic avian influenza A(H5N1) is ongoing; health care providers and persons with exposure to sick or infected birds should remain vigilant for onset of symptoms consistent with influenza. Receiving a seasonal influenza vaccine each year remains the best way to protect against seasonal influenza and its potentially severe consequences.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Emergency preparedness and response: highly pathogenic avian influenza A(H5N1) virus: recommendations for human health investigations and response. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://emergency.cdc.gov/han/2022/han00464.asp
-
- CDC. Influenza (flu): U.S. influenza surveillance: purpose and methods. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. Accessed May 27, 2022. https://www.cdc.gov/flu/weekly/overview.htm
-
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2022–2023 northern hemisphere influenza season. Geneva, Switzerland: World Health Organization; 2022. https://cdn.who.int/media/docs/default-source/influenza/who-influenza-re...
-
- Food and Drug Administration. Summary minutes: 171st Vaccines and Related Biological Products Advisory Committee. Silver Spring, MD: US Department of Health and Human Serivices, Food and Drug Administration; 2022. https://www.fda.gov/media/157170/download
-
- CDC. Influenza (flu): 2021–2022 U.S. flu season: preliminary in-season burden estimates. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Accessed May 27, 2022. https://www.cdc.gov/flu/about/burden/preliminary-in-season-estimates.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

