A cardiac-null mutation of Prdm16 causes hypotension in mice with cardiac hypertrophy via increased nitric oxide synthase 1
- PMID: 35862303
- PMCID: PMC9302805
- DOI: 10.1371/journal.pone.0267938
A cardiac-null mutation of Prdm16 causes hypotension in mice with cardiac hypertrophy via increased nitric oxide synthase 1
Abstract
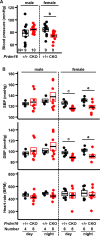
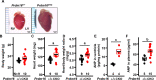
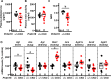
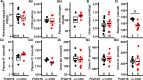
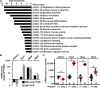
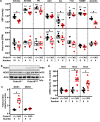
Hypertension or hypotension prevails as a comorbidity in patients with heart failure (HF). Although blood pressure (BP) is an important factor in managing the mortality of HF, the molecular mechanisms of changes in BP have not been clearly understood in cases of HF. We and others have demonstrated that a loss in PRDM16 causes hypertrophic cardiomyopathy, leading to HF. We aimed to determine whether BP is altered in mice that experience cardiac loss of Prdm16 and identify the underlying mechanism of BP-associated changes. BP decreased significantly only in female mice with a cardiac-null mutation of Prdm16 compared with controls, by an invasive protocol under anesthesia and by telemetric method during conscious, unrestrained status. Mice with a cardiac loss of Prdm16 had higher heart-to-body weight ratios and upregulated atrial natriuretic peptide, suggesting cardiac hypertrophy. Plasma aldosterone-to-renin activity ratios and plasma sodium levels decreased in Prdm16-deficient mice versus control. By RNA-seq and in subsequent functional analyses, Prdm16-null hearts were enriched in factors that regulate BP, including Adra1a, Nos1, Nppa, and Nppb. The inhibition of nitric oxide synthase 1 (NOS1) reverted the decrease in BP in cardiac-specific Prdm16 knockout mice. Mice with cardiac deficiency of Prdm16 present with hypotension and cardiac hypertrophy. Further, our findings suggest that the increased expression of NOS1 causes hypotension in mice with a cardiac-null mutation of Prdm16. These results provide novel insights into the molecular mechanisms of hypotension in subjects with HF and contribute to our understanding of how hypotension develops in patients with HF.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al.. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–63. doi: 10.1016/s0195-668x(02)00823-0 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

