Exercise and hypoxia unmask pulmonary vascular disease and right ventricular dysfunction in a 10- to 12-week-old swine model of neonatal oxidative injury
- PMID: 35862359
- PMCID: PMC9542957
- DOI: 10.1113/JP282906
Exercise and hypoxia unmask pulmonary vascular disease and right ventricular dysfunction in a 10- to 12-week-old swine model of neonatal oxidative injury
Abstract
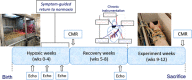
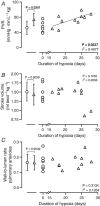
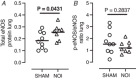
Prematurely born young adults who experienced neonatal oxidative injury (NOI) of the lungs have increased incidence of cardiovascular disease. Here, we investigated the long-term effects of NOI on cardiopulmonary function in piglets at the age of 10-12 weeks. To induce NOI, term-born piglets (1.81 ± 0.06 kg) were exposed to hypoxia (10-12% ), within 2 days after birth, and maintained for 4 weeks or until symptoms of heart failure developed (range 16-28 days), while SHAM piglets were normoxia raised. Following recovery (>5 weeks), NOI piglets were surgically instrumented to measure haemodynamics during hypoxic challenge testing (HCT) and exercise with modulation of the nitric-oxide system. During exercise, NOI piglets showed a normal increase in cardiac index, but an exaggerated increase in pulmonary artery pressure and a blunted increase in left atrial pressure - suggesting left atrial under-filling - consistent with an elevated pulmonary vascular resistance (PVR), which correlated with the duration of hypoxia exposure. Moreover, hypoxia duration correlated inversely with stroke volume (SV) during exercise. Nitric oxide synthase inhibition and HCT resulted in an exaggerated increase in PVR, while the PVR reduction by phosphodiesterase-5 inhibition was enhanced in NOI compared to SHAM piglets. Finally, within the NOI piglet group, prolonged duration of hypoxia was associated with a better maintenance of SV during HCT, likely due to the increase in RV mass. In conclusion, duration of neonatal hypoxia appears an important determinant of alterations in cardiopulmonary function that persist further into life. These changes encompass both pulmonary vascular and cardiac responses to hypoxia and exercise. KEY POINTS: Children who suffered from neonatal oxidative injury, such as very preterm born infants, have increased risk of cardiopulmonary disease later in life. Risk stratification requires knowledge of the mechanistic underpinning and the time course of progression into cardiopulmonary disease. Exercise and hypoxic challenge testing showed that 10- to 12-week-old swine that previously experienced neonatal oxidative injury had increased pulmonary vascular resistance and nitric oxide dependency. Duration of neonatal oxidative injury was a determinant of structural and functional cardiopulmonary remodelling later in life. Remodelling of the right ventricle, as a result of prolonged neonatal oxidative injury, resulted in worse performance during exercise, but enabled better performance during the hypoxic challenge test. Increased nitric oxide dependency together with age- or comorbidity-related endothelial dysfunction may contribute to predisposition to pulmonary hypertension later in life.
Keywords: heart failure; neonatal; pulmonary vascular disease.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None.
Figures




Comment in
-
Hypoxic heart hypertrophy: an indepth examination of cardiac remodelling using a swine model of neonatal oxidative injury.J Physiol. 2023 Jul;601(14):2761-2762. doi: 10.1113/JP284622. Epub 2023 May 11. J Physiol. 2023. PMID: 37145094 No abstract available.
Similar articles
-
Structural and functional changes of the pulmonary vasculature after hypoxia exposure in the neonatal period: a new swine model of pulmonary vascular disease.Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H603-H615. doi: 10.1152/ajpheart.00362.2017. Epub 2017 Nov 10. Am J Physiol Heart Circ Physiol. 2018. PMID: 29127236
-
Cardiac remodelling in a swine model of chronic thromboembolic pulmonary hypertension: comparison of right vs. left ventricle.J Physiol. 2019 Sep;597(17):4465-4480. doi: 10.1113/JP277896. Epub 2019 Jul 25. J Physiol. 2019. PMID: 31194256 Free PMC article.
-
Changes in the nitric oxide pathway of the pulmonary vasculature after exposure to hypoxia in swine model of neonatal pulmonary vascular disease.Physiol Rep. 2018 Oct;6(20):e13889. doi: 10.14814/phy2.13889. Physiol Rep. 2018. PMID: 30375198 Free PMC article.
-
Endogenous nitric oxide and pulmonary vascular tone in the neonate.J Pediatr Surg. 1996 Jan;31(1):137-40. doi: 10.1016/s0022-3468(96)90336-x. J Pediatr Surg. 1996. PMID: 8632267
-
The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension.Acta Physiol (Oxf). 2017 Apr;219(4):728-756. doi: 10.1111/apha.12749. Epub 2016 Jul 31. Acta Physiol (Oxf). 2017. PMID: 27381367 Review.
Cited by
-
Long-Term Adverse Effects of Perinatal Hypoxia on the Adult Pulmonary Circulation Vary Between Males and Females in a Murine Model.Physiol Res. 2024 Nov 29;73(S2):S541-S556. doi: 10.33549/physiolres.935481. Physiol Res. 2024. PMID: 39589302 Free PMC article.
-
Cardiac dysfunction during exercise in young adults with bronchopulmonary dysplasia.ERJ Open Res. 2024 Jun 17;10(3):00501-2023. doi: 10.1183/23120541.00501-2023. eCollection 2024 May. ERJ Open Res. 2024. PMID: 38887679 Free PMC article.
References
-
- Abman, S. H. , Kinsella, J. P. , Rosenzweig, E. B. , Krishnan, U. , Kulik, T. , Mullen, M. , Wessel, D. L. , Steinhorn, R. , Adatia, I. , Hanna, B. , Feinstein, J. , Fineman, J. , Raj, U. , & Humpl, T. , & Pediatric Pulmonary Hypertension N (2013). Implications of the U.S. Food and Drug Administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 187(6), 572–575. - PubMed
-
- Backhaus, S. J. , Lange, T. , George, E. F. , Hellenkamp, K. , Gertz, R. J. , Billing, M. , Wachter, R. , Steinmetz, M. , Kutty, S. , Raaz, U. , Lotz, J. , Friede, T. , Uecker, M. , Hasenfuss, G. , Seidler, T. , & Schuster, A. (2021). Exercise‐stress real‐time cardiac magnetic resonance imaging for non‐invasive characterisation of heart failure with preserved ejection fraction: The HFpEF stress trial. Circulation, 143(15), 1484–1498. - PubMed
-
- Bates, M. L. , Levy, P. T. , Nuyt, A. M. , Goss, K. N. , Lewandowski, A. J. , & McNamara, P. J. (2020). Adult Cardiovascular health risk and cardiovascular phenotypes of prematurity. Journal of Pediatrics, 227, 17–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

