LRBA is essential for urinary concentration and body water homeostasis
- PMID: 35862451
- PMCID: PMC9335246
- DOI: 10.1073/pnas.2202125119
LRBA is essential for urinary concentration and body water homeostasis
Abstract
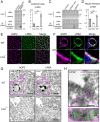
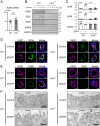
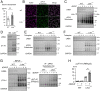
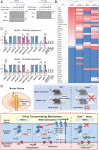
Protein kinase A (PKA) directly phosphorylates aquaporin-2 (AQP2) water channels in renal collecting ducts to reabsorb water from urine for the maintenance of systemic water homeostasis. More than 50 functionally distinct PKA-anchoring proteins (AKAPs) respectively create compartmentalized PKA signaling to determine the substrate specificity of PKA. Identification of an AKAP responsible for AQP2 phosphorylation is an essential step toward elucidating the molecular mechanisms of urinary concentration. PKA activation by several compounds is a novel screening strategy to uncover PKA substrates whose phosphorylation levels were nearly perfectly correlated with that of AQP2. The leading candidate in this assay proved to be an AKAP termed lipopolysaccharide-responsive and beige-like anchor protein (LRBA). We found that LRBA colocalized with AQP2 in vivo, and Lrba knockout mice displayed a polyuric phenotype with severely impaired AQP2 phosphorylation. Most of the PKA substrates other than AQP2 were adequately phosphorylated by PKA in the absence of LRBA, demonstrating that LRBA-anchored PKA preferentially phosphorylated AQP2 in renal collecting ducts. Furthermore, the LRBA-PKA interaction, rather than other AKAP-PKA interactions, was robustly dissociated by PKA activation. AKAP-PKA interaction inhibitors have attracted attention for their ability to directly phosphorylate AQP2. Therefore, the LRBA-PKA interaction is a promising drug target for the development of anti-aquaretics.
Keywords: AKAP; AQP2; LRBA; PKA; urinary concentration.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Identification of protein kinase A signalling molecules in renal collecting ducts.J Physiol. 2024 Jul;602(13):3057-3067. doi: 10.1113/JP284178. Epub 2023 Apr 17. J Physiol. 2024. PMID: 37013848 Review.
-
LRBA signalosomes activate vasopressin-induced AQP2 trafficking at recycling endosomes.J Physiol. 2023 Dec;601(23):5437-5451. doi: 10.1113/JP285188. Epub 2023 Oct 20. J Physiol. 2023. PMID: 37860942
-
Activation of AQP2 water channels by protein kinase A: therapeutic strategies for congenital nephrogenic diabetes insipidus.Clin Exp Nephrol. 2021 Oct;25(10):1051-1056. doi: 10.1007/s10157-021-02108-6. Epub 2021 Jul 5. Clin Exp Nephrol. 2021. PMID: 34224008 Free PMC article. Review.
-
Cyclin-Dependent Kinase 18 Controls Trafficking of Aquaporin-2 and Its Abundance through Ubiquitin Ligase STUB1, Which Functions as an AKAP.Cells. 2020 Mar 10;9(3):673. doi: 10.3390/cells9030673. Cells. 2020. PMID: 32164329 Free PMC article.
-
Lipopolysaccharide-responsive beige-like anchor acts as a cAMP-dependent protein kinase anchoring protein in B cells.Scand J Immunol. 2020 Sep;92(3):e12922. doi: 10.1111/sji.12922. Epub 2020 Jul 14. Scand J Immunol. 2020. PMID: 32592188
Cited by
-
Methods for studying mammalian aquaporin biology.Biol Methods Protoc. 2023 Nov 11;8(1):bpad031. doi: 10.1093/biomethods/bpad031. eCollection 2023. Biol Methods Protoc. 2023. PMID: 38046463 Free PMC article. Review.
-
Goreisan promotes diuresis by regulating the abundance of aquaporin 2 phosphorylated at serine 269 through calcium-sensing receptor activation.Sci Rep. 2024 Nov 28;14(1):29650. doi: 10.1038/s41598-024-81324-y. Sci Rep. 2024. PMID: 39609591 Free PMC article.
-
Lipopolysaccharide-responsive beige-like anchor is involved in regulating NF-κB activation in B cells.Front Immunol. 2024 Jul 15;15:1409434. doi: 10.3389/fimmu.2024.1409434. eCollection 2024. Front Immunol. 2024. PMID: 39076990 Free PMC article.
-
Role of Protein Kinase A Activation in the Immune System with an Emphasis on Lipopolysaccharide-Responsive and Beige-like Anchor Protein in B Cells.Int J Mol Sci. 2023 Feb 4;24(4):3098. doi: 10.3390/ijms24043098. Int J Mol Sci. 2023. PMID: 36834508 Free PMC article. Review.
-
LRBA, a BEACH protein mutated in human immune deficiency, is widely expressed in epithelia, exocrine and endocrine glands, and neurons.Sci Rep. 2024 May 9;14(1):10678. doi: 10.1038/s41598-024-60257-6. Sci Rep. 2024. PMID: 38724551 Free PMC article.
References
-
- Sands J. M., Bichet D. G., Physicians A. C., Society A. P.; American College of Physicians; American Physiological Society, Nephrogenic diabetes insipidus. Ann. Intern. Med. 144, 186–194 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

