Mass screening is a key component to fight against SARS-CoV-2 and return to normalcy
- PMID: 35862506
- PMCID: PMC9274759
- DOI: 10.1515/mr-2021-0024
Mass screening is a key component to fight against SARS-CoV-2 and return to normalcy
Abstract
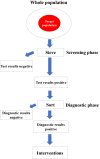
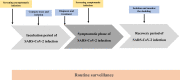
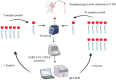
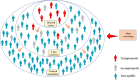
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had highly transmissible and pathogenic, which caused serious economic loss and hazard to public health. Different countries have developed strategies to deal with the COVID-19 pandemic that fit their epidemiological situations, capacities, and values. Mass screening combined with control measures rapidly reduced the transmission of the SARS-CoV-2 infection. The COVID-19 pandemic has dramatically highlighted the essential role of diagnostics capacity in the control of communicable diseases. Mass screening has been increasingly used to detect suspected COVID-19 cases and their close contacts, asymptomatic case, patients attending fever clinics, high-risk populations, employees, even all population to identify infectious individuals. Mass screening is a key component to fight against SARS-CoV-2 and return to normalcy. Here we describe the history of mass screening, define the scope of mass screening, describe its application scenarios, and discuss the impact and challenges of using this approach to control COVID-19. We conclude that through a comprehension screening program and strong testing capabilities, mass screening could help us return to normalcy more quickly.
Keywords: SARS-CoV-2; close contacts; diagnostics capacity; mass screening.
© 2022 Zhaomin Feng et al., published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures
Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Epidemiological and viral features of a cohort of SARS-CoV-2 symptomatic and asymptomatic individuals in an area of the Colombian Caribbean.Ann Clin Microbiol Antimicrob. 2020 Dec 7;19(1):58. doi: 10.1186/s12941-020-00397-5. Ann Clin Microbiol Antimicrob. 2020. PMID: 33287846 Free PMC article.
-
The Positive Rate of Nucleic Acid Testing and the Epidemiological Characteristics of COVID-19 in Chongqing.Front Med (Lausanne). 2022 Jan 14;8:802708. doi: 10.3389/fmed.2021.802708. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35096891 Free PMC article.
-
The role of respiratory microbiota in the protection against viral diseases: respiratory commensal bacteria as next-generation probiotics for COVID-19.Biosci Microbiota Food Health. 2022;41(3):94-102. doi: 10.12938/bmfh.2022-009. Epub 2022 Mar 29. Biosci Microbiota Food Health. 2022. PMID: 35846832 Free PMC article. Review.
-
Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms.Cells. 2022 Mar 31;11(7):1182. doi: 10.3390/cells11071182. Cells. 2022. PMID: 35406746 Free PMC article. Review.
Cited by
-
A proactive/reactive mass screening approach with uncertain symptomatic cases.PLoS Comput Biol. 2024 Aug 14;20(8):e1012308. doi: 10.1371/journal.pcbi.1012308. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39141678 Free PMC article.
-
Effectiveness of inactivated COVID-19 vaccines in preventing COVID-19-related hospitalization during the Omicron BF.7-predominant epidemic wave in Beijing, China: a cohort study.BMC Infect Dis. 2024 Sep 17;24(1):991. doi: 10.1186/s12879-024-09889-7. BMC Infect Dis. 2024. PMID: 39289630 Free PMC article.
-
The Prevalence of COVID-19 in Europe by the End of November 2022: A Cross-Sectional Study.Cureus. 2023 Jan 9;15(1):e33546. doi: 10.7759/cureus.33546. eCollection 2023 Jan. Cureus. 2023. PMID: 36779135 Free PMC article.
-
Aptamer-based diagnosis of various SARS-CoV2 strains isolated from clinical specimens.Heliyon. 2023 Jun;9(6):e16458. doi: 10.1016/j.heliyon.2023.e16458. Epub 2023 May 23. Heliyon. 2023. PMID: 37251485 Free PMC article.
-
Integrating Magnetic-Bead-Based Sample Extraction and Molecular Barcoding for the One-Step Pooled RT-qPCR Assay of Viral Pathogens without Retesting.Anal Chem. 2023 Apr 11;95(14):6182-6190. doi: 10.1021/acs.analchem.3c00885. Epub 2023 Apr 2. Anal Chem. 2023. PMID: 37005794 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
