Comprehensive narrative review of real-world COVID-19 vaccines: viewpoints and opportunities
- PMID: 35862507
- PMCID: PMC9274757
- DOI: 10.1515/mr-2021-0021
Comprehensive narrative review of real-world COVID-19 vaccines: viewpoints and opportunities
Abstract
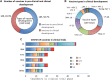
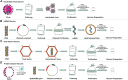
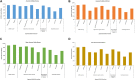
Currently, people all over the world have been affected by coronavirus disease 2019 (COVID-19). Fighting against COVID-19 is the top priority for all the countries and nations. The development of a safe and effective COVID-19 vaccine is considered the optimal way of ending the pandemic. Three hundred and 44 vaccines were in development, with 149 undergoing clinical research and 35 authorized for emergency use as to March 15 of 2022. Many studies have shown the effective role of COVID-19 vaccines in preventing SARS-CoV-2 infections as well as serious and fatal COVID-19 cases. However, tough challenges have arisen regarding COVID-19 vaccines, including long-term immunity, emerging COVID-19 variants, and vaccine inequalities. A systematic review was performed of recent COVID-19 vaccine studies, with a focus on vaccine type, efficacy and effectiveness, and protection against SARS-CoV-2 variants, breakthrough infections, safety, deployment and vaccine strategies used in the real-world. Ultimately, there is a need to establish a unified evaluation standard of vaccine effectiveness, monitor vaccine safety and effectiveness, along with the virological characteristics of SARS-CoV-2 variants; and determine the most useful booster schedule. These aspects must be coordinated to ensure timely responses to beneficial or detrimental situations. In the future, global efforts should be directed toward effective and immediate vaccine allocations, improving vaccine coverage, SARS-CoV-2 new variants tracking, and vaccine booster development.
Keywords: SARS-CoV-2 variants; breakthrough infection; coronavirus disease 2019; emergency use authorization; mass vaccine administration; vaccine type.
© 2022 Shelan Liu et al., published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures



Similar articles
-
COVID-19 vaccines: their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants.Bull Natl Res Cent. 2022;46(1):96. doi: 10.1186/s42269-022-00787-z. Epub 2022 Apr 8. Bull Natl Res Cent. 2022. PMID: 35431535 Free PMC article. Review.
-
COVID-19 vaccines: concerns beyond protective efficacy and safety.Expert Rev Vaccines. 2021 Aug;20(8):1013-1025. doi: 10.1080/14760584.2021.1949293. Epub 2021 Jul 5. Expert Rev Vaccines. 2021. PMID: 34180347 Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021.MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1163-1166. doi: 10.15585/mmwr.mm7034e3. MMWR Morb Mortal Wkly Rep. 2021. PMID: 34437519 Free PMC article.
-
Analysis of COVID-19 Incidence and Severity Among Adults Vaccinated With 2-Dose mRNA COVID-19 or Inactivated SARS-CoV-2 Vaccines With and Without Boosters in Singapore.JAMA Netw Open. 2022 Aug 1;5(8):e2228900. doi: 10.1001/jamanetworkopen.2022.28900. JAMA Netw Open. 2022. PMID: 36018588 Free PMC article.
Cited by
-
COVID-19 severity and vaccine effectiveness in Malawi: A test-negative case-control study.J Public Health Afr. 2025 Apr 11;16(1):758. doi: 10.4102/jphia.v16i1.758. eCollection 2025. J Public Health Afr. 2025. PMID: 40356739 Free PMC article.
-
Serological Response to SARS-CoV-2 after COVID-19 Vaccination in Lung Cancer Patients: Short Review.Vaccines (Basel). 2023 May 11;11(5):969. doi: 10.3390/vaccines11050969. Vaccines (Basel). 2023. PMID: 37243073 Free PMC article. Review.
-
Characteristics and Outcomes for Recipients of NVX-CoV2373: A Real-World Retrospective Study in Germany.Vaccines (Basel). 2024 Apr 6;12(4):387. doi: 10.3390/vaccines12040387. Vaccines (Basel). 2024. PMID: 38675769 Free PMC article.
-
A Review of Inactivated COVID-19 Vaccine Development in China: Focusing on Safety and Efficacy in Special Populations.Vaccines (Basel). 2023 May 31;11(6):1045. doi: 10.3390/vaccines11061045. Vaccines (Basel). 2023. PMID: 37376434 Free PMC article. Review.
-
Current Status and Factors of Vaccine Hesitancy in Tetanus Vaccination Among Traumatic Patients - China, 2024.China CDC Wkly. 2025 Mar 28;7(13):441-448. doi: 10.46234/ccdcw2025.071. China CDC Wkly. 2025. PMID: 40226523 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
