The α- and β-Subunit Boundary at the Stem of the Mushroom-Like α3β3-Type Oxygenase Component of Rieske Non-Heme Iron Oxygenases Is the Rieske-Type Ferredoxin-Binding Site
- PMID: 35862661
- PMCID: PMC9361823
- DOI: 10.1128/aem.00835-22
The α- and β-Subunit Boundary at the Stem of the Mushroom-Like α3β3-Type Oxygenase Component of Rieske Non-Heme Iron Oxygenases Is the Rieske-Type Ferredoxin-Binding Site
Abstract
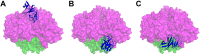
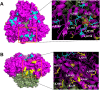
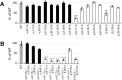
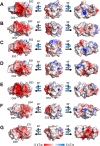
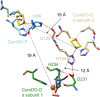
Cumene dioxygenase (CumDO) is an initial enzyme in the cumene degradation pathway of Pseudomonas fluorescens IP01 and is a Rieske non-heme iron oxygenase (RO) that comprises two electron transfer components (reductase [CumDO-R] and Rieske-type ferredoxin [CumDO-F]) and one catalytic component (α3β3-type oxygenase [CumDO-O]). Catalysis is triggered by electrons that are transferred from NAD(P)H to CumDO-O by CumDO-R and CumDO-F. To investigate the binding mode between CumDO-F and CumDO-O and to identify the key CumDO-O amino acid residues for binding, we simulated docking between the CumDO-O crystal structure and predicted model of CumDO-F and identified two potential binding sites: one is at the side-wise site and the other is at the top-wise site in mushroom-like CumDO-O. Then, we performed alanine mutagenesis of 16 surface amino acid residues at two potential binding sites. The results of reduction efficiency analyses using the purified components indicated that CumDO-F bound at the side-wise site of CumDO-O, and K117 of the α-subunit and R65 of the β-subunit were critical for the interaction. Moreover, these two positively charged residues are well conserved in α3β3-type oxygenase components of ROs whose electron donors are Rieske-type ferredoxins. Given that these residues were not conserved if the electron donors were different types of ferredoxins or reductases, the side-wise site of the mushroom-like structure is thought to be the common binding site between Rieske-type ferredoxin and α3β3-type oxygenase components in ROs. IMPORTANCE We clarified the critical amino acid residues of the oxygenase component (Oxy) of Rieske non-heme iron oxygenase (RO) for binding with Rieske-type ferredoxin (Fd). Our results showed that Rieske-type Fd-binding site is commonly located at the stem (side-wise site) of the mushroom-like α3β3 quaternary structure in many ROs. The resultant binding site was totally different from those reported at the top-wise site of the doughnut-like α3-type Oxy, although α3-type Oxys correspond to the cap (α3 subunit part) of the mushroom-like α3β3-type Oxys. Critical amino acid residues detected in this study were not conserved if the electron donors of Oxys were different types of Fds or reductases. Altogether, we can suggest that unique binding modes between Oxys and electron donors have evolved, depending on the nature of the electron donors, despite Oxy molecules having shared α3β3 quaternary structures.
Keywords: Rieske non-heme iron oxygenase; dioxygenases; electron transport; ferredoxin; protein-protein interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Specific Interactions between the ferredoxin and terminal oxygenase components of a class IIB Rieske nonheme iron oxygenase, carbazole 1,9a-dioxygenase.J Mol Biol. 2009 Sep 18;392(2):436-51. doi: 10.1016/j.jmb.2009.07.029. Epub 2009 Jul 17. J Mol Biol. 2009. PMID: 19616558
-
Structural investigations of the ferredoxin and terminal oxygenase components of the biphenyl 2,3-dioxygenase from Sphingobium yanoikuyae B1.BMC Struct Biol. 2007 Mar 9;7:10. doi: 10.1186/1472-6807-7-10. BMC Struct Biol. 2007. PMID: 17349044 Free PMC article.
-
Structure of the terminal oxygenase component of angular dioxygenase, carbazole 1,9a-dioxygenase.J Mol Biol. 2005 Aug 12;351(2):355-70. doi: 10.1016/j.jmb.2005.05.059. J Mol Biol. 2005. PMID: 16005887
-
Rieske business: structure-function of Rieske non-heme oxygenases.Biochem Biophys Res Commun. 2005 Dec 9;338(1):175-90. doi: 10.1016/j.bbrc.2005.08.222. Epub 2005 Sep 8. Biochem Biophys Res Commun. 2005. PMID: 16168954 Review.
-
Rieske Oxygenases and Other Ferredoxin-Dependent Enzymes: Electron Transfer Principles and Catalytic Capabilities.Chembiochem. 2023 Aug 1;24(15):e202300078. doi: 10.1002/cbic.202300078. Epub 2023 Jun 21. Chembiochem. 2023. PMID: 36964978 Review.
Cited by
-
Engineering Rieske oxygenase activity one piece at a time.Curr Opin Chem Biol. 2023 Feb;72:102227. doi: 10.1016/j.cbpa.2022.102227. Epub 2022 Nov 18. Curr Opin Chem Biol. 2023. PMID: 36410250 Free PMC article. Review.
-
Understanding the stability of a plastic-degrading Rieske iron oxidoreductase system.Protein Sci. 2024 Jun;33(6):e4997. doi: 10.1002/pro.4997. Protein Sci. 2024. PMID: 38723110 Free PMC article.
-
Analysis of Benzoate 1,2-Dioxygenase Identifies Shared Electron Transfer Components With DxnA1A2 in Rhizorhabdus wittichii RW1.J Basic Microbiol. 2025 Aug;65(8):e70061. doi: 10.1002/jobm.70061. Epub 2025 May 22. J Basic Microbiol. 2025. PMID: 40405529 Free PMC article.
-
Functional and spectroscopic approaches to determining thermal limitations of Rieske oxygenases.Methods Enzymol. 2024;703:299-328. doi: 10.1016/bs.mie.2024.05.021. Epub 2024 Jun 29. Methods Enzymol. 2024. PMID: 39261001 Free PMC article.
References
-
- Cerniglia CE. 1993. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Cell Biol 4:331–338. 10.1016/0958-1669(93)90104-5. - DOI
-
- Gibson DT, Subramanian Z. 1984. Microbial degradation of aromatic compounds, p 181–252. In Gibson DT (ed), Microbial degradation of organic compounds. Marcel Dekker, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

