Genome Analysis of Enterobacter asburiae and Lelliottia spp. Proliferating in Oligotrophic Drinking Water Reservoirs and Lakes
- PMID: 35862664
- PMCID: PMC9317948
- DOI: 10.1128/aem.00471-22
Genome Analysis of Enterobacter asburiae and Lelliottia spp. Proliferating in Oligotrophic Drinking Water Reservoirs and Lakes
Abstract
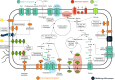
Surface waters are one of the main sources for drinking water production, and thus microbial contamination should be as minimal as possible. However, high concentrations of coliform bacteria were detected in reservoirs and lakes used for drinking water production during summer months due to autochthonous proliferation processes. Here, we present the genomic analyses of 17 strains of Enterobacter asburiae and Lelliottia spp. proliferating in reservoirs and lakes with special focus on the hygienic relevance, antibiotic resistance, and adaptations to the oligotrophic environments. The genomes contain neither genes for the type III secretion system nor cytotoxins or hemolysins, which are considered typical virulence factors. Examination of antibiotic resistance genes revealed mainly efflux pumps and β-lactamase class C (ampC) genes. Phenotypically, single isolates of Enterobacter asburiae showed resistance to fosfomycin and ceftazidime. The genome analyses further suggest adaptations to oligotrophic and changing environmental conditions in reservoirs and lakes, e.g., genes to cope with low nitrate and phosphate levels and the ability to utilize substances released by algae, like amino acids, chitin, alginate, rhamnose, and fucose. This leads to the hypothesis that the proliferation of the coliform bacteria could occur at the end of summer due to algae die-off. IMPORTANCE Certain strains of coliform bacteria have been shown to proliferate in the oligotrophic water of drinking water reservoirs and lakes, reaching values above 104 per 100 mL. Such high concentrations challenge drinking water treatment, and occasionally the respective coliform bacteria have been detected in the treated drinking water. Thus, the question of their hygienic relevance is of high importance for water suppliers and authorities. Our genomic analyses suggest that the strains are not hygienically relevant, as typical virulence factors are absent and antibiotic resistance genes in the genomes most likely are of natural origin. Furthermore, their presence in the water is not related to fecal contamination. The proliferation in reservoirs and lakes during stable summer stratification is an autochthonic process of certain E. asburiae and Lelliottia strains that are well adapted to the surrounding oligotrophic environment.
Keywords: Enterobacter; Lelliottia; drinking water; hygienic relevance; lakes; oligotrophic waters; reservoir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Exner M, Behringer K, Wernicke F, Dautzenberg W. 2005. Erfahrungen mit coliformen—befunden in einem trinkwasser-talsperrensystem in Nordrhein-Westfalen, p 15–24. In Arbeitsgemeinschaft Trinkwassertalsperren e.V. (ATT) (ed), Coliformen-befunde gemäß Trinkwasserversordnung 2001. Bewertung und maßnahmen, vol 5. Kommissionsverlag Oldenbourg Industrieverlag GmbH, Siegburg.
-
- Freier K, Feuerpfeil I, Hummel A, Schulze H, Buder G. 2005. Erfahrungen mit coliformen-befunden an trinkwassertalsperren in Sachsen, p 25–36. In Arbeitsgemeinschaft Trinkwassertalsperren e.V. (ATT) (ed), Coliformen-befunde gemäß trinkwasserversordnung 2001. Bewertung und maßnahmen, vol 5. Kommissionsverlag Oldenbourg Industrieverlag GmbH, Siegburg.
-
- Packroff G, Clasen J. 2005. Eine Massenentwicklung von Enterobacter asburiae im Wasser der Wahnbachtalsperre im September 2003, p 37–54. In Arbeitsgemeinschaft Trinkwassertalsperren e.V. (ATT) (ed), Coliformen-befunde gemäß trinkwasserversordnung 2001. Bewertung und maßnahmen, vol 5. Kommissionsverlag Oldenbourg Industrieverlag GmbH, Siegburg.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

