Regulation of l- and d-Aspartate Transport and Metabolism in Acinetobacter baylyi ADP1
- PMID: 35862682
- PMCID: PMC9361831
- DOI: 10.1128/aem.00883-22
Regulation of l- and d-Aspartate Transport and Metabolism in Acinetobacter baylyi ADP1
Abstract
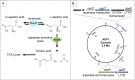
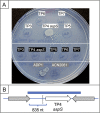
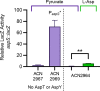
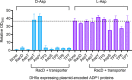
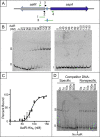
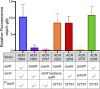
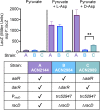
The regulated uptake and consumption of d-amino acids by bacteria remain largely unexplored, despite the physiological importance of these compounds. Unlike other characterized bacteria, such as Escherichia coli, which utilizes only l-Asp, Acinetobacter baylyi ADP1 can consume both d-Asp and l-Asp as the sole carbon or nitrogen source. As described here, two LysR-type transcriptional regulators (LTTRs), DarR and AalR, control d- and l-Asp metabolism in strain ADP1. Heterologous expression of A. baylyi proteins enabled E. coli to use d-Asp as the carbon source when either of two transporters (AspT or AspY) and a racemase (RacD) were coexpressed. A third transporter, designated AspS, was also discovered to transport Asp in ADP1. DarR and/or AalR controlled the transcription of aspT, aspY, racD, and aspA (which encodes aspartate ammonia lyase). Conserved residues in the N-terminal DNA-binding domains of both regulators likely enable them to recognize the same DNA consensus sequence (ATGC-N7-GCAT) in several operator-promoter regions. In strains lacking AalR, suppressor mutations revealed a role for the ClpAP protease in Asp metabolism. In the absence of the ClpA component of this protease, DarR can compensate for the loss of AalR. ADP1 consumed l- and d-Asn and l-Glu, but not d-Glu, as the sole carbon or nitrogen source using interrelated pathways. IMPORTANCE A regulatory scheme was revealed in which AalR responds to l-Asp and DarR responds to d-Asp, a molecule with critical signaling functions in many organisms. The RacD-mediated interconversion of these isomers causes overlap in transcriptional control in A. baylyi. Our studies improve understanding of transport and regulation and lay the foundation for determining how regulators distinguish l- and d-enantiomers. These studies are relevant for biotechnology applications, and they highlight the importance of d-amino acids as natural bacterial growth substrates.
Keywords: ADP1; Acinetobacter baylyi; DarR; LTTR; LysR; aspartate; racemase; regulation; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

