A Virion-Based Combination Vaccine Protects against Influenza and SARS-CoV-2 Disease in Mice
- PMID: 35862698
- PMCID: PMC9364787
- DOI: 10.1128/jvi.00689-22
A Virion-Based Combination Vaccine Protects against Influenza and SARS-CoV-2 Disease in Mice
Abstract
Vaccines targeting SARS-CoV-2 have been shown to be highly effective; however, the breadth against emerging variants and the longevity of protection remains unclear. Postimmunization boosting has been shown to be beneficial for disease protection, and as new variants continue to emerge, periodic (and perhaps annual) vaccination will likely be recommended. New seasonal influenza virus vaccines currently need to be developed every year due to continual antigenic drift, an undertaking made possible by a robust global vaccine production and distribution infrastructure. To create a seasonal combination vaccine targeting both influenza viruses and SARS-CoV-2 that is also amenable to frequent reformulation, we have developed an influenza A virus (IAV) genetic platform that allows the incorporation of an immunogenic domain of the SARS-CoV-2 spike (S) protein onto IAV particles. Vaccination with this combination vaccine elicited neutralizing antibodies and provided protection from lethal challenge with both pathogens in mice. This approach may allow the leveraging of established influenza vaccine infrastructure to generate a cost-effective and scalable seasonal vaccine solution for both influenza and coronaviruses. IMPORTANCE The rapid emergence of SARS-CoV-2 variants since the onset of the pandemic has highlighted the need for both periodic vaccination "boosts" and a platform that can be rapidly reformulated to manufacture new vaccines. In this work, we report an approach that can utilize current influenza vaccine manufacturing infrastructure to generate combination vaccines capable of protecting from both influenza virus- and SARS-CoV-2-induced disease. The production of a combined influenza/SARS-CoV-2 vaccine may represent a practical solution to boost immunity to these important respiratory viruses without the increased cost and administration burden of multiple independent vaccines.
Keywords: SARS-CoV-2; influenza vaccines.
Conflict of interest statement
The authors declare a conflict of interest. Duke University has filed for intellectual property protection of the approaches in this manuscript.
Figures
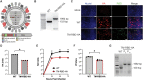
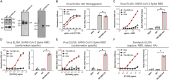
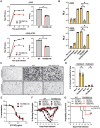
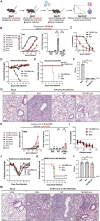

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- G20-AI167200/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- 75N93019C00050/AI/NIAID NIH HHS/United States
- T32 CA009111/CA/NCI NIH HHS/United States
- HHSN272201400005C/AI/NIAID NIH HHS/United States
- UC6-AI058607/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

