Mycoplasma pneumoniae Compared to Streptococcus pneumoniae Avoids Induction of Proinflammatory Epithelial Cell Responses despite Robustly Inducing TLR2 Signaling
- PMID: 35862703
- PMCID: PMC9387261
- DOI: 10.1128/iai.00129-22
Mycoplasma pneumoniae Compared to Streptococcus pneumoniae Avoids Induction of Proinflammatory Epithelial Cell Responses despite Robustly Inducing TLR2 Signaling
Abstract
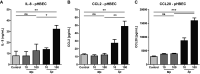
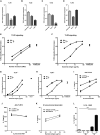
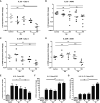
Mycoplasma pneumoniae and Streptococcus pneumoniae are the most common bacterial causes of pneumonia in children. The clinical characteristics of pneumonia differ significantly between the two bacteria. We aimed to elucidate the differences in pathogenesis between M. pneumoniae and S. pneumoniae by characterizing the respiratory epithelial cell immune response to both pathogens. Using primary human bronchial epithelial cells in air-liquid interface cultures, we observed lower production of the proinflammatory cytokines interleukin-6 (IL-6) and IL-8 in response to M. pneumoniae than to S. pneumoniae. In contrast to the differences in proinflammatory cytokine production, Toll-like receptor 2 (TLR2)-mediated signaling in response to M. pneumoniae was stronger than to S. pneumoniae. This difference largely depended on TLR1 and not TLR6. We found that M. pneumoniae, but not S. pneumoniae, also induced signaling of TLR10, a coreceptor of TLR2 that has inhibitory properties. M. pneumoniae-induced TLR10 signaling on airway epithelial cells was partially responsible for low IL-8 production, as blocking TLR10 by specific antibodies increased cytokine production. M. pneumoniae maintained Th2-associated cytokine production by epithelial cells, which concurs with the known association of M. pneumoniae infection with asthma. M. pneumoniae left IL-33 levels unchanged, whereas S. pneumoniae downregulated IL-33 production both under homeostatic and Th2-promoting conditions. By directly comparing M. pneumoniae and S. pneumoniae, we demonstrate that M. pneumoniae avoids induction of proinflammatory cytokine response despite its ability to induce robust TLR2 signaling. Our new findings suggest that this apparent paradox may be partially explained by M. pneumoniae-induced signaling of TLR2/TLR10.
Keywords: IL-33; IL-8; Mycoplasma pneumoniae; Streptococcus pneumoniae; TLR10; TLR2; host response; pneumonia; primary bronchial epithelial cells; respiratory pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization. 2016. Pneumonia fact sheet. World Health Organization, Geneva, Switzerland. Accessed 16 April 2018.
-
- Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, Zhu Y, Patel A, Hymas W, Chappell JD, Kaufman RA, Kan JH, Dansie D, Lenny N, Hillyard DR, Haynes LM, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, Wunderink RG, Edwards KM, Pavia AT, McCullers JA, Finelli L, Team CES. 2015. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 372:835–845. doi:10.1056/NEJMoa1405870. - DOI - PMC - PubMed
-
- Meyer Sauteur PM, Krautter S, Ambroggio L, Seiler M, Paioni P, Relly C, Capaul R, Kellenberger C, Haas T, Gysin C, Bachmann LM, van Rossum AMC, Berger C. 2020. Improved diagnostics help to identify clinical features and biomarkers that predict Mycoplasma pneumoniae community-acquired pneumonia in children. Clin Infect Dis 71:1645–1654. doi:10.1093/cid/ciz1059. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

