Expression of CD117 (c-Kit) on Circulating B Cells in Pediatric Schistosomiasis
- PMID: 35862720
- PMCID: PMC9387214
- DOI: 10.1128/iai.00160-22
Expression of CD117 (c-Kit) on Circulating B Cells in Pediatric Schistosomiasis
Abstract
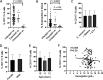
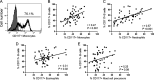
Few B cells express CD27, the primary marker for memory B cells, in pediatric schistosomiasis, suggesting B cell malfunction. This study further demonstrates unexpected high expression of CD117 on circulating B cells in children highly exposed to Schistosoma mansoni infectious larvae. CD117 is expressed by immature or lymphoma B cells, but not by mature, circulating cells. We therefore sought to define the significance of CD117 on blood B cells. We found that CD117-positive (CD117+) B cells increased with the intensity of schistosome infection. In addition, CD117 expression was reduced on CD23+ B cells previously shown to correlate with resistance to infection. Stimulation with a panel of cytokines demonstrated that CD117 levels were upregulated in response to a combination of interleukin 4 (IL-4) and stem cell factor (SCF), the ligand for CD117, whereas IL-2 led to a reduction. In addition, stimulation with SCF generally reduced B cell activation levels. Upon further investigation, it was established that multiple circulating cells expressed increased levels of CD117, including monocytes, neutrophils, and eosinophils, and expression levels correlated with that of B cells. Finally, we identified a population of large circulating cells with features of reticulocytes. Overall, our results suggest that hyperexposure to intravascular parasitic worms elicits immature cells from the bone marrow. Levels of SCF were shown to reduce as children began to transition through puberty. The study results pose an explanation for the inability of children to develop significant immunity to infection until after puberty.
Keywords: B cells; CD117; CD23; pediatric schistosomiasis; schistosomiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



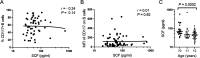

References
-
- Handzel T, Hightower AW, Colley DG, Rosen DH, Andove J, Karanja DMS, Addiss DG, Slutsker L, Secor WE. 2003. Geographic distribution of schistosomiasis and soil-transmitted helminths in Western Kenya: implications for anthelminthic mass treatment. Am J Trop Med Hyg 69:318–323. doi:10.4269/ajtmh.2003.69.318. - DOI - PubMed
-
- Onkanga IO, Mwinzi PNM, Muchiri G, Andiego K, Omedo M, Karanja DMS, Wiegand RE, Secor WE, Montgomery SP. 2016. Impact of two rounds of praziquantel mass drug administration on Schistosoma mansoni infection prevalence and intensity: a comparison between community wide treatment and school based treatment in western Kenya. Int J Parasitol 46:439–445. doi:10.1016/j.ijpara.2016.01.006. - DOI - PMC - PubMed
-
- Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, Fulford AJC. 1999. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology 118:101–105. doi:10.1017/S0031182098003576. - DOI - PubMed
-
- Vereecken K, Naus CWA, Polman K, Scott JT, Diop M, Gryseels B, Kestens L. 2007. Associations between specific antibody responses and resistance to reinfection in a Senegalese population recently exposed to Schistosoma mansoni. Trop Med Int Health 12:431–444. doi:10.1111/j.1365-3156.2006.01805.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

