Controlled Human Infection Models To Accelerate Vaccine Development
- PMID: 35862754
- PMCID: PMC9491212
- DOI: 10.1128/cmr.00008-21
Controlled Human Infection Models To Accelerate Vaccine Development
Abstract
The timelines for developing vaccines against infectious diseases are lengthy, and often vaccines that reach the stage of large phase 3 field trials fail to provide the desired level of protective efficacy. The application of controlled human challenge models of infection and disease at the appropriate stages of development could accelerate development of candidate vaccines and, in fact, has done so successfully in some limited cases. Human challenge models could potentially be used to gather critical information on pathogenesis, inform strain selection for vaccines, explore cross-protective immunity, identify immune correlates of protection and mechanisms of protection induced by infection or evoked by candidate vaccines, guide decisions on appropriate trial endpoints, and evaluate vaccine efficacy. We prepared this report to motivate fellow scientists to exploit the potential capacity of controlled human challenge experiments to advance vaccine development. In this review, we considered available challenge models for 17 infectious diseases in the context of the public health importance of each disease, the diversity and pathogenesis of the causative organisms, the vaccine candidates under development, and each model's capacity to evaluate them and identify correlates of protective immunity. Our broad assessment indicated that human challenge models have not yet reached their full potential to support the development of vaccines against infectious diseases. On the basis of our review, however, we believe that describing an ideal challenge model is possible, as is further developing existing and future challenge models.
Keywords: controlled human infection model; human challenge model; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
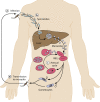
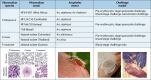


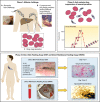

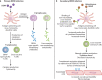




References
-
- Geenen V. 2020. Claude Bernard (1813–1878), the father of modern physiology and experimental medicine. http://orbi.ulg.ac.be/bitstream/2268/79049/1/C.BERNARD%20Text.pdf. Accessed 28 May 2022.
-
- World Health Organization. 2020. World malaria report 2020. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240015791.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

