Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Meropenem in Neurocritical Care Patients: a Prospective Two-Center Study
- PMID: 35862757
- PMCID: PMC9380572
- DOI: 10.1128/aac.00142-22
Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Meropenem in Neurocritical Care Patients: a Prospective Two-Center Study
Abstract
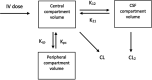
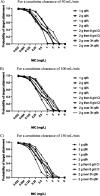
Morbidity and mortality related to ventriculitis in neurocritical care patients remain high. Antibiotic dose optimization may improve therapeutic outcomes. In this study, a population pharmacokinetic model of meropenem in infected critically ill patients was developed. We applied the final model to determine optimal meropenem dosing regimens required to achieve targeted cerebrospinal fluid exposures. Neurocritical care patients receiving meropenem and with a diagnosis of ventriculitis or extracranial infection were recruited from two centers to this study. Serial plasma and cerebrospinal fluid samples were collected and assayed. Population pharmacokinetic modeling and Monte Carlo dosing simulations were performed using Pmetrics. We sought to determine optimized dosing regimens that achieved meropenem cerebrospinal fluid concentrations above pathogen MICs for 40% of the dosing interval, or a higher target ratio of meropenem cerebrospinal fluid trough concentrations to pathogen MIC of ≥1. In total, 53 plasma and 34 cerebrospinal fluid samples were obtained from eight patients. Meropenem pharmacokinetics were appropriately described using a three-compartment model with linear plasma clearance scaled for creatinine clearance and cerebrospinal fluid penetration scaled for patient age. Considerable interindividual pharmacokinetic variability was apparent, particularly in the cerebrospinal fluid. Percent coefficients of variation for meropenem clearance from plasma and cerebrospinal fluid were 41.7% and 89.6%, respectively; for meropenem, the volume of distribution in plasma and cerebrospinal fluid values were 63.4% and 58.3%, respectively. High doses (up to 8 to 10 g/day) improved attainment of meropenem cerebrospinal fluid target exposures, particularly for less susceptible organisms (MICs, ≥0.25 mg/L). Standard meropenem doses of 2 g every 8 h may not achieve effective concentrations in cerebrospinal fluid in all critically ill patients. Higher doses, or alternative dosing methods (e.g., loading dose followed by continuous infusion) may be required to optimize cerebrospinal fluid exposures. Doses of up to 8 to 10 g/day either as intermittent boluses or continuous infusion would be suitable for patients with augmented renal clearance; lower doses may be considered for patients with impaired renal function as empirical suggestions. Ongoing dosing should be tailored to the individual patient circumstances. Notably, the study population was small and dosing recommendations may not be generalizable to all critically ill patients.
Keywords: CSF; carbapenem; dosing; pharmacodynamics; pharmacokinetics; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kumta N, Roberts JA, Lipman J, Wong WT, Joynt GM, Cotta MO. 2020. A systematic review of studies reporting antibiotic pharmacokinetic data in the cerebrospinal fluid of critically ill patients with uninflamed meninges. Antimicrob Agents Chemother 65:e01998-20. 10.1128/AAC.01998-20. - DOI - PMC - PubMed
-
- Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL, International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases . 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 14:498–509. 10.1016/S1473-3099(14)70036-2. - DOI - PMC - PubMed
-
- Mouton J. 2016. General concepts of pharmacodynamics for anti-infective agents. In Rotschafer J, Andes D, Rodvold K (ed), Antibiotic pharmacodynamics. Springer, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

