Impact of Prior Infection on SARS-CoV-2 Antibody Responses in Vaccinated Long-Term Care Facility Staff
- PMID: 35862798
- PMCID: PMC9429942
- DOI: 10.1128/msphere.00169-22
Impact of Prior Infection on SARS-CoV-2 Antibody Responses in Vaccinated Long-Term Care Facility Staff
Abstract
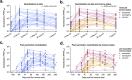
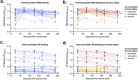
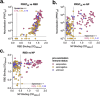
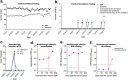
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in 2019 and has resulted in millions of deaths worldwide. Certain populations are at higher risk for infection, especially staff and residents at long-term care facilities (LTCF), due to the congregant living setting and high proportions of residents with many comorbidities. Prior to vaccine availability, these populations represented large fractions of total coronavirus disease 2019 (COVID-19) cases and deaths in the United States. Due to the high-risk setting and outbreak potential, staff and residents were among the first groups to be vaccinated. To define the impact of prior infection on the response to vaccination, we measured antibody responses in a cohort of staff members at an LTCF, many of whom were previously infected by SARS-CoV-2. We found that neutralizing, receptor-binding domain (RBD)-binding, and nucleoprotein (NP)-binding antibody levels were significantly higher after the full vaccination course in individuals that were previously infected and that NP antibody levels could discriminate individuals with prior infection from vaccinated individuals. While an anticipated antibody titer increase was observed after a vaccine booster dose in naive individuals, a boost response was not observed in individuals with previous COVID-19 infection. We observed a strong relationship between neutralizing antibodies and RBD-binding antibodies postvaccination across all groups, whereas no relationship was observed between NP-binding and neutralizing antibodies. One individual with high levels of neutralizing and binding antibodies experienced a breakthrough infection (prior to the introduction of Omicron), demonstrating that the presence of antibodies is not always sufficient for complete protection against infection. These results highlight that a history of COVID-19 exposure significantly increases SARS-CoV-2 antibody responses following vaccination. IMPORTANCE Long-term care facilities (LTCFs) have been disproportionately impacted by COVID-19, due to their communal nature, the high-risk profile of residents, and the vulnerability of residents to respiratory pathogens. In this study, we analyzed the role of prior natural immunity to SARS-CoV-2 in postvaccination antibody responses. The LTCF in our cohort experienced a large outbreak, with almost 40% of staff members becoming infected. We found that individuals that were infected prior to vaccination had higher levels of neutralizing and binding antibodies postvaccination. Importantly, the second vaccine dose significantly boosted antibody levels in those that were immunologically naive prior to vaccination, but not in those that had prior immunity. Regardless of the prevaccination immune status, the levels of binding and neutralizing antibodies were highly correlated. The presence of NP-binding antibodies could be used to identify individuals that were previously infected when prevaccination immune status was not known. Our results reveal that vaccination antibody responses differ depending on prior natural immunity.
Keywords: COVID-19; SARS-CoV-2; correlate of protection; neutralizing antibodies; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The New York Times. 16 February 2022. Coronavirus in the U.S.: latest map and case count. https://www.nytimes.com/interactive/2021/us/covid-cases.html.
-
- Centers for Medicare & Medicaid Services. 16 February 2022. COVID-19 nursing home data. https://data.cms.gov/covid-19/covid-19-nursing-home-data. - PubMed
-
- Ochieng N, Chidambaram P, Garfield R, Neuman T. 14 January 2021. Factors associated with COVID-19 cases and deaths in long-term care facilities: findings from a literature review. Kaiser Family Foundation, San Francisco, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
