Tight Complex Formation of the Fumarate Sensing DcuS-DcuR Two-Component System at the Membrane and Target Promoter Search by Free DcuR Diffusion
- PMID: 35862816
- PMCID: PMC9429925
- DOI: 10.1128/msphere.00235-22
Tight Complex Formation of the Fumarate Sensing DcuS-DcuR Two-Component System at the Membrane and Target Promoter Search by Free DcuR Diffusion
Abstract
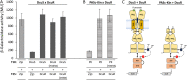
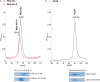
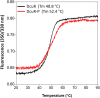
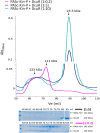
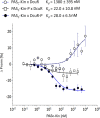
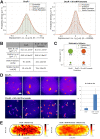
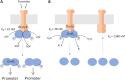
Signaling of two-component systems by phosphoryl transfer requires interaction of the sensor kinase with the response regulator. Interaction of the C4-dicarboxylate-responsive and membrane-integral sensor kinase DcuS with the response regulator DcuR was studied. In vitro, the cytoplasmic part of DcuS (PASC-Kin) was employed. Stable complexes were formed, when either DcuS or DcuR were phosphorylated (Kd 22 ± 11 and 28 ± 7 nM, respectively). The unphosphorylated proteins produced a more labile complex (Kd 1380 ± 395 nM). Bacterial two-hybrid studies confirm interaction of DcuR with DcuS (and PASC-Kin) in vivo. The absolute contents of DcuR (197-979 pmol mg-1 protein) in the bacteria exceeded those of DcuS by more than 1 order of magnitude. According to the Kd values, DcuS exists in complex, with phosphorylated but also unphosphorylated DcuR. In live cell imaging, the predominantly freely diffusing DcuR becomes markedly less mobile after phosphorylation and activation of DcuS by fumarate. Portions of the low mobility fraction accumulated at the cell poles, the preferred location of DcuS, and other portions within the cell, representing phosphorylated DcuR bound to promoters. In the model, acitvation of DcuS increases the affinity toward DcuR, leading to DcuS-P × DcuR formation and phosphorylation of DcuR. The complex is stable enough for phosphate-transfer, but labile enough to allow exchange between DcuR from the cytosol and DcuR-P of the complex. Released DcuR-P diffuses to target promoters and binds. Uncomplexed DcuR-P in the cytosol binds to nonactivated DcuS and becomes dephosphorylated. The lower affinity between DcuR and DcuS avoids blocking of DcuS and allows rapid exchange of DcuR. IMPORTANCE Complex formation of membrane-bound sensor kinases with the response regulators represents an inherent step of signaling from the membrane to the promoters on the DNA. In the C4-dicarboxylate-sensing DcuS-DcuR two-component system, complex formation is strengthened by activation (phosphorylation) in vitro and in vivo, with trapping of the response regulator DcuR at the membrane. Single-molecule tracking of DcuR in the bacterial cell demonstrates two populations of DcuR with decreased mobility in the bacteria after activation: one at the membrane, but a second in the cytosol, likely representing DNA-bound DcuR. The data suggest a model with binding of DcuR to DcuS-P for phosphorylation, and of DcuR-P to DcuS for dephosphorylation, allowing rapid adaptation of the DcuR phosphorylation state. DcuR-P is released and transferred to DNA by 3D diffusion.
Keywords: DcuS-DcuR; bacteria; complex formation; sensor complex; sensor kinase; two-component system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
