Simple, Rapid Chemical Labeling and Screening of Antibodies with Luminescent Peptides
- PMID: 35862857
- PMCID: PMC9396617
- DOI: 10.1021/acschembio.2c00306
Simple, Rapid Chemical Labeling and Screening of Antibodies with Luminescent Peptides
Abstract
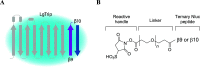
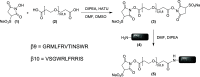
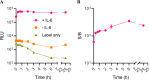
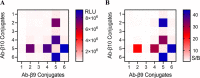
Sensitive and selective detection assays are essential for the accurate measurement of analytes in both clinical and research laboratories. Immunoassays that rely on nonoverlapping antibodies directed against the same target analyte (e.g., sandwich enzyme-linked immunosorbent assays (ELISAs)) are commonly used molecular detection technologies. Use of split enzyme reporters has simplified the workflow for these traditionally complex assays. However, identifying functional antibody pairs for a given target analyte can be cumbersome, as it generally involves generating and screening panels of antibodies conjugated to reporters. Accordingly, we sought a faster and easier reporter conjugation strategy to streamline antibody screening. We describe here the development of such a method that is based on an optimized ternary NanoLuc luciferase. This bioluminescence complementation system is comprised of a reagent-based thermally stable polypeptide (LgTrip) and two small peptide tags (β9 and β10) with lysine-reactive handles for direct conjugation onto antibodies. These reagents enable fast, single-step, wash-free antibody labeling and sensitive functional screening. Simplicity, speed, and utility of the one-pot labeling technology are demonstrated in screening antibody pairs for the analyte interleukin-4. The screen resulted in the rapid development of a sensitive homogeneous immunoassay for this clinically relevant cytokine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Development of a rapid, simple, and sensitive point-of-care technology platform utilizing ternary NanoLuc.Front Microbiol. 2022 Oct 26;13:970233. doi: 10.3389/fmicb.2022.970233. eCollection 2022. Front Microbiol. 2022. PMID: 36386626 Free PMC article.
-
Toward a Point-of-Need Bioluminescence-Based Immunoassay Utilizing a Complete Shelf-Stable Reagent.Anal Chem. 2021 Mar 30;93(12):5177-5184. doi: 10.1021/acs.analchem.0c05074. Epub 2021 Mar 17. Anal Chem. 2021. PMID: 33730483
-
Enhanced nanobody-driven bioluminescent immunoassay for rapid parathion detection using engineered split-nanoluciferase.Biosens Bioelectron. 2025 Feb 1;269:116913. doi: 10.1016/j.bios.2024.116913. Epub 2024 Nov 8. Biosens Bioelectron. 2025. PMID: 39549312
-
Immunoanalysis methods for the detection of dioxins and related chemicals.Sensors (Basel). 2012 Dec 5;12(12):16710-31. doi: 10.3390/s121216710. Sensors (Basel). 2012. PMID: 23443395 Free PMC article. Review.
-
Multi-analyte immunoassay.J Pharm Biomed Anal. 1989;7(2):155-68. doi: 10.1016/0731-7085(89)80079-2. J Pharm Biomed Anal. 1989. PMID: 2488616 Review.
Cited by
-
Rapid and Wash-Free Anti-Drug Antibody Assay Enabled by a Complementary Nanoluciferase Biosensor.Anal Chem. 2025 Jul 15;97(27):14273-14280. doi: 10.1021/acs.analchem.5c01017. Epub 2025 Jul 2. Anal Chem. 2025. PMID: 40601873
-
CRISPR RiPCA for Investigating eIF4E-m7GpppX Capped mRNA Interactions.bioRxiv [Preprint]. 2025 Jun 22:2025.06.19.660603. doi: 10.1101/2025.06.19.660603. bioRxiv. 2025. Update in: ACS Chem Biol. 2025 Aug 15;20(8):2038-2048. doi: 10.1021/acschembio.5c00471. PMID: 40666878 Free PMC article. Updated. Preprint.
-
Development of Luminescent Biosensors for Calprotectin.ACS Chem Biol. 2024 Jun 21;19(6):1250-1259. doi: 10.1021/acschembio.4c00265. Epub 2024 Jun 6. ACS Chem Biol. 2024. PMID: 38843544 Free PMC article.
-
Development of a rapid, simple, and sensitive point-of-care technology platform utilizing ternary NanoLuc.Front Microbiol. 2022 Oct 26;13:970233. doi: 10.3389/fmicb.2022.970233. eCollection 2022. Front Microbiol. 2022. PMID: 36386626 Free PMC article.
-
Split-Protein Therapeutic Platforms: Identifying Binder Pairs.Methods Mol Biol. 2024;2720:75-84. doi: 10.1007/978-1-0716-3469-1_5. Methods Mol Biol. 2024. PMID: 37775658
References
-
- Cox K. L.; Devanarayan V.; Kriauciunas A.; Manetta J.; Montrose C.; Sittampalam S.. Immunoassay Methods. In Assay Guidance Manual; Markossian S.; Grossman A.; Brimacombe K.; Arkin M.; Auld D.; Austin C. P.; Baell J.; Chung T. D. Y.; Coussens N. P.; Dahlin J. L.. et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2004. - PubMed
-
- Hall M. P.; Unch J.; Binkowski B. F.; Valley M. P.; Butler B. L.; Wood M. G.; Otto P.; Zimmerman K.; Vidugiris G.; Machleidt T.; et al. Engineered Luciferase Reporter from a Deep Sea Shrimp Utilizing a Novel Imidazopyrazinone Substrate. ACS Chem. Biol. 2012, 7, 1848–1857. 10.1021/cb3002478. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

