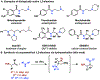
Synthesis of Unsymmetrical Vicinal Diamines via Directed Hydroamination
- PMID: 35862860
- PMCID: PMC9757009
- DOI: 10.1021/acs.orglett.2c01911
Synthesis of Unsymmetrical Vicinal Diamines via Directed Hydroamination
Abstract
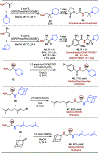
Vicinal diamines are a common motif found in biologically active molecules. The hydroamination of allyl amine derivatives is a powerful approach for the synthesis of substituted 1,2-diamines. Herein, the rhodium-catalyzed hydroamination of primary and secondary allylic amines using diverse amine nucleophiles, including primary, secondary, acyclic, and cyclic aliphatic amines to access a wide range of unsymmetrical vicinal diamines, is presented. The utility of this methodology is further demonstrated through the rapid synthesis of several bioactive molecules and analogs.
Figures
Similar articles
-
Rhodium-Catalyzed Asymmetric Hydroamination of Allyl Amines.J Am Chem Soc. 2019 Jan 16;141(2):739-742. doi: 10.1021/jacs.8b09811. Epub 2019 Jan 7. J Am Chem Soc. 2019. PMID: 30614700 Free PMC article.
-
Rhodium-/Iridium-Catalyzed Hydroamination for the Synthesis of 1,2-, 1,3-, or 1,4-Diamines.ACS Catal. 2022 Jul 15;12(14):8331-8340. doi: 10.1021/acscatal.2c01835. Epub 2022 Jun 28. ACS Catal. 2022. PMID: 37143789 Free PMC article.
-
Regio- and Enantioselective Synthesis of 1,2-Diamine Derivatives by Copper-Catalyzed Hydroamination.Org Lett. 2019 Jun 7;21(11):4370-4373. doi: 10.1021/acs.orglett.9b01592. Epub 2019 May 17. Org Lett. 2019. PMID: 31099584 Free PMC article.
-
Asymmetric Rhodium-Catalyzed Allylic Substitution Reactions: Discovery, Development and Applications to Target-Directed Synthesis.J Org Chem. 2018 Oct 5;83(19):11463-11479. doi: 10.1021/acs.joc.8b00583. Epub 2018 Sep 5. J Org Chem. 2018. PMID: 30183287 Review.
-
Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes.Angew Chem Int Ed Engl. 2016 Jan 4;55(1):48-57. doi: 10.1002/anie.201507594. Epub 2015 Dec 10. Angew Chem Int Ed Engl. 2016. PMID: 26661678 Free PMC article. Review.
Cited by
-
Iridium-catalysed hydroamination of internal homoallylic amines.Chem Commun (Camb). 2024 Feb 6;60(12):1615-1618. doi: 10.1039/d3cc05594a. Chem Commun (Camb). 2024. PMID: 38230687 Free PMC article.
-
Ring Opening of Aziridines by Pendant Sulfamates Allows for Regioselective and Stereospecific Preparation of Vicinal Diamines.J Org Chem. 2023 Nov 17;88(22):15989-16006. doi: 10.1021/acs.joc.3c01731. Epub 2023 Oct 30. J Org Chem. 2023. PMID: 37903411 Free PMC article.
-
Synthesis of Diazacyclic and Triazacyclic Small-Molecule Libraries Using Vicinal Chiral Diamines Generated from Modified Short Peptides and Their Application for Drug Discovery.Pharmaceuticals (Basel). 2024 Nov 22;17(12):1566. doi: 10.3390/ph17121566. Pharmaceuticals (Basel). 2024. PMID: 39770408 Free PMC article. Review.
-
[3 + 2] Cycloadditions of Tertiary Amine N-Oxides and Silyl Imines as an Innovative Route to 1,2-Diamines.Org Lett. 2023 Jun 30;25(25):4638-4643. doi: 10.1021/acs.orglett.3c01396. Epub 2023 Jun 15. Org Lett. 2023. PMID: 37318514 Free PMC article.
References
-
- Ricci A, Amino group chemistry: from synthesis to the life sciences, Wiley-VCH, Weinhein, 2008;
- Lucet D; Gall TL; Mioskowski C The Chemistry of Vicinal Diamines. Angew. Chem. Int. Ed 1998, 37, 2580–2627. - PubMed
-
- Harrington RA; Hamilton CW; Brogden RN; Linkewich JA; Romankiewicz JA; Heel RC Metoclopramide. An updated review of its pharmacological properties and clinical use. Drugs, 1983, 25, 451–494. - PubMed
- Koch-Weser J; Klein SW Procainamide Dosage Schedules, Plasma Concentrations, and Clinical Effects. JAMA 1971, 215, 1454–1460. - PubMed
- Bonnet U Moclobemide: therapeutic use and clinical studies. CNS Drug Rev. 2003, 9, 97–140. - PMC - PubMed
- Hollingshead LM; Faulds D; Fitton A Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Drugs 1992, 44, 835–857. - PubMed
- Ouellet D; Sutherland S; Wang T; Griffini P; Murthy V First-time-in-human study with GSK1018921, a selective GlyT1 inhibitor: relationship between exposure and dizziness. Clin. Pharmacol. Ther 2011, 90, 597–604. - PubMed
- Carosati E; Cruciani G; Chiarini A; Budriesi R; Ioan P; Spisani R; Spinelli D; Cosimelli B; Devlin FJ Calcium Channel Antagonists Discovered by a Multidisciplinary Approach. J. Med. Chem 2006, 49, 5206–5216. - PubMed
- Sacksteder KA; Protopopova M; Barry CE III; Andries KK; Nacy CA Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol. 2012, 7, 823–837. - PMC - PubMed
-
- Fristad WE; Brandvold TA; Peterson JR; Thompson SR Conversion of alkenes to 1,2-diazides and 1,2-diamines. J. Org. Chem 1985, 50, 3647–3649.
- Zhu Y; Cornwall RG; Du H; Zhao B; Shi Y Catalytic Diamination of Olefins via N–N Bond Activation. Acc. Chem. Res 2014, 47, 3665–3678. - PMC - PubMed
- Olson DE; Su JY; Roberts DA; Du Bois J Vicinal Diamination of Alkenes under Rh-Catalysis. J. Am. Chem. Soc 2014, 136, 13506–13509. - PMC - PubMed
- Fumagalli G; Rabet PTG; Boyd S; Greaney BF Three-Component Azidation of Styrene-Type Double Bonds: Light-Switchable Behavior of a Copper Photoredox Catalyst. Angew. Chem. Int. Ed 2015, 54, 11481–11484. - PMC - PubMed
- Lu M-Z; Wang C-Q; Loh T-P Copper-Catalyzed Vicinal Oxyazidation and Diazidation of Styrenes under Mild Conditions: Access to Alkyl Azides. Org. Lett 2015, 17, 6110–6113. - PubMed
- Yuan YA; Lu D-F; Chen YR; Xu H Iron-Catalyzed Direct Diazidation for a Broad Range of Olefins. Angew. Chem. Int. Ed 2016, 55, 534–538. - PMC - PubMed
- Muñiz KK,; Martínez C; Iglesias A The Quest for Palladium-Catalysed Alkyl–Nitrogen Bond Formation. Chem. Rec 2016, 16, 2561–2572. - PubMed
- Shen S-J; Zhu C-L; Lu D-F; Xu H Iron-Catalyzed Direct Olefin Diazidation via Peroxyester Activation Promoted by Nitrogen-Based Ligands. ACS Catal. 2018, 8, 4473–4482. - PMC - PubMed
- Muñiz K; Barreiro L; Romero RM; Martínez C Catalytic Asymmetric Diamination of Styrenes. J. Am. Chem. Soc 2017, 139, 4354–4357. - PubMed
- Fu N; Sauer GS; Saha A; Loo A; Lin S Metal-catalyzed electrochemical diazidation of alkenes. Science 2017, 357, 575–579. - PubMed
- Cai C-Y; Shu X-M; Xu H-C Practical and stereoselective electrocatalytic 1,2-diamination of alkenes. Nat. Commun 2019, 10, 4953. - PMC - PubMed
- Govaerts S; Angelini L; Hampton C; Malet-Sanz L; Ruffoni A; Leonori D Photoinduced Olefin Diamination with Alkylamines. Angew. Chem. Int. Ed 2020, 59, 15021–15028. - PMC - PubMed
- Li Y; Ali A; Dong J; Zhang Y; Shi L; Liu Q; Fu J Copper-Catalyzed Diamination of Unactivated Alkenes With Electron-Rich Amino Sources. Org. Lett 2021, 23, 4072–4077. - PubMed
-
- Ickes AR; Ensign SC; Gupta AK; Hull KL Regio- and Chemoselective Intermolecular Hydroamination of Allyl Imines for the Synthesis of 1,2-Diamines. J. Am. Chem. Soc 2014, 136, 11256–11259. - PubMed
- Vanable EP; Kennemur JL; Joyce LA; Ruck RT; Schultz DM; Hull KL Rhodium-Catalyzed Asymmetric Hydroamination of Allyl Amines. J. Am. Chem. Soc 2019, 141, 739–742. - PMC - PubMed
- Zhao S-B; Bilodeau E; Lemieux V; Beauchemin AM Hydrogen Bonding Directed Intermolecular Cope-Type Hydroamination of Alkenes. Org. Lett 2012, 14, 5081–5085. - PubMed
- Hesp CR; MacDonald MJ; Zahedi MM; Bilodeau DA; Zhao S-B; Pesant M; Beauchemin AM Formaldehyde as Tethering Organocatalyst: Highly Diastereoselective Hydroaminations of Allylic Amines. Org. Lett 2015, 17, 5136–5139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources