Generating Heterokaryotic Cells via Bacterial Cell-Cell Fusion
- PMID: 35862998
- PMCID: PMC9430406
- DOI: 10.1128/spectrum.01693-22
Generating Heterokaryotic Cells via Bacterial Cell-Cell Fusion
Abstract
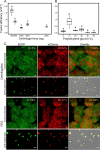
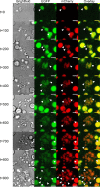
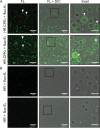
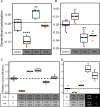
Fusion of cells is an important and common biological process that leads to the mixing of cellular contents and the formation of multinuclear cells. Cell fusion occurs when distinct membranes are brought into proximity of one another and merge to become one. Fusion holds promise for biotechnological innovations, for instance, for the discovery of urgently needed new antibiotics. Here, we used antibiotic-producing bacteria that can proliferate without their cell wall as a model to investigate cell-cell fusion. We found that fusion between genetically distinct cells yields heterokaryons that are viable, contain multiple selection markers, and show increased antimicrobial activity. The rate of fusion induced using physical and chemical methods was dependent on membrane fluidity, which is related to lipid composition as a function of cellular age. Finally, by using an innovative system of synthetic membrane-associated lipopeptides, we achieved targeted fusion between distinctly marked cells to further enhance fusion efficiency. These results provide a molecular handle to understand and control cell-cell fusion, which can be used in the future for the discovery of new drugs. IMPORTANCE Cell-cell fusion is instrumental in introducing different sets of genes in the same environment, which subsequently leads to diversity. There is need for new protocols to fuse cells of different types together for biotechnological applications like drug discovery. We present here wall-deficient cells as a platform for the same. We identify the fluidity of the membrane as an important characteristic for the process of fusion. We demonstrate a cell-specific approach for fusion using synthetically designed peptides yielding cells with modified antibiotic production profiles. Overall, wall-deficient cells can be a chassis for innovative metabolite production by providing an alternative method for cell-cell fusion.
Keywords: cell fusion; cell membranes; cell wall deficient; coiled-coil peptides; heterokaryon; lipopeptides; membrane fluidity; protoplast fusion; wall deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

