Human Air-Liquid-Interface Organotypic Airway Cultures Express Significantly More ACE2 Receptor Protein and Are More Susceptible to HCoV-NL63 Infection than Monolayer Cultures of Primary Respiratory Epithelial Cells
- PMID: 35863002
- PMCID: PMC9431431
- DOI: 10.1128/spectrum.01639-22
Human Air-Liquid-Interface Organotypic Airway Cultures Express Significantly More ACE2 Receptor Protein and Are More Susceptible to HCoV-NL63 Infection than Monolayer Cultures of Primary Respiratory Epithelial Cells
Abstract
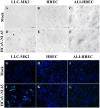
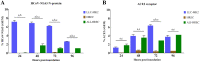
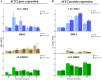
Human coronavirus NL63 (HCoV-NL63) is commonly associated with mild respiratory tract infections in infants, being that the respiratory epithelial cells are the main target for infection and initial replication of this virus. Standard immortalized cells are highly permissive to HCoV-NL63, and they are routinely used for isolation and propagation of the virus from clinical specimens. However, these cell lines are not the natural cell target of the virus and lack sufficient complexity to mimic the natural infection process in vivo. This study comparatively evaluated the differences on the susceptibility to HCoV-NL63 infection and virus replication efficiency of submerged monolayer cultures of LLC-MK2 and primary human respiratory epithelial cells (HRECs) and organotypic airway cultures of respiratory cells (ALI-HRECs). Productive viral infection and growth kinetics were assessed by morphologic examination of cytopathic effects, immunofluorescence, reverse transcription quantitative real-time PCR, and flow cytometry. Results from this study showed higher susceptibility to HCoV-NL63 infection and replication in LLC-MK2 cells followed by ALI-HRECs, with very low susceptibility and no significant virus replication in HRECs. This susceptibility was associated with the expression levels of angiontensin-converting enzyme 2 (ACE2) receptor protein in LLC-MK2, ALI-HRECs, and HRECs, respectively. Remarkably, organotypic ALI-HREC cultures expressed significantly more ACE2 receptor protein and were more susceptible to HCoV-NL63 infection than monolayer cultures of HREC. The ACE2 receptor is, therefore, a critical factor for susceptibility to HCoV-NL63 infection and replication, as is the type of culture used during infection studies. IMPORTANCE HCoV-NL63 is widespread globally, accounting for a significant number of respiratory infections in children and adults. HCoV-NL63 gains entrance into respiratory epithelial cells via the ACE2 receptor, the same cell receptor used by severe acute respiratory syndrome coronavirus (SARS-CoV) and SARS-CoV-2. Thus, HCoV-NL63 has been suggested as safe surrogate for studying disease mechanisms and therapeutic interventions against SARS-like CoVs, while working under BSL-2 conditions. The present study not only showed the critical role of ACE2 for effective HCoV-NL63 infection and replication, but also shed light on the need of more refined and complex in vitro organotypic models that recapitulate the proxy of air-liquid respiratory epithelia cell composition, structure, and functionality. These cultures have broaden virological studies toward improving our understanding of how coronaviruses cause disease and transmission not just within humans but also in animal populations.
Keywords: ACE2; Alphacoronavirus; HCoV-NL63; LLC-MK2; coronavirus; infection; organotypic airway cultures; primary human respiratory epithelial cells; upper respiratory tract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- International Committee on Taxonomy of Viruses (ICTV). 2020. ICTV taxonomy history: human coronavirus NL63. https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=20200.... Accessed May 3, 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

