Compartment and Plant Identity Shape Tree Mycobiome in a Subtropical Forest
- PMID: 35863008
- PMCID: PMC9430249
- DOI: 10.1128/spectrum.01347-22
Compartment and Plant Identity Shape Tree Mycobiome in a Subtropical Forest
Abstract
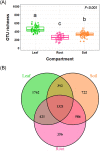
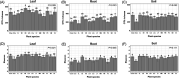
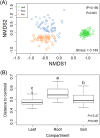
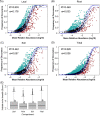
Deciphering the relationships between microbes and their host plants is critical for a better understanding of microbial diversity maintenance and community stability. Here, we investigated fungal diversity and community assembly in the phyllosphere and rhizosphere of 13 tree species in a subtropical common-garden experiment. The results showed that fungal community structures significantly differed across compartments (leaf, root, and soil) and different tree species. Higher α-diversity was observed in the phyllosphere than in the roots and rhizospheric soil. Fungal community composition (β-diversity) was significantly affected by both compartment and species identity. The fungal community compositions were significantly correlated with soil pH in the roots and the soils as well as with soil nitrate and leaf total phosphorus in the leaves. We found that fungal community assemblies were mainly driven by deterministic processes, regardless of compartments. Moreover, host preference analyses indicated that stronger plant/fungus preferences occurred in leaves than in roots and soils. Our results highlight the differences in tree mycobiome between aboveground and belowground compartments and have important implications for the promotion of biodiversity conservation and management sustainability for the subtropical forest. IMPORTANCE Subtropical mountain forests are widely distributed in Southern China and are characterized by high biodiversity. The interactions between plants and fungi play pivotal roles in biodiversity maintenance and community stability. Nevertheless, knowledge of fungal diversity and of the community assembly patterns of woody plants is scarce. Here, we investigated fungal diversity and community assembly in the phyllosphere and rhizosphere of 13 tree species in a common-garden experiment. We found that both compartment and plant identity influenced fungal diversity, community, and guild compositions, while deterministic processes mainly governed the fungal community assembly, especially in the rhizospheric fungal communities. Our results demonstrate that tree leaves represent stronger host/fungi preferences than do roots and soils. Together, our findings enhance the understanding of the roles of compartment and plant identity in structuring fungal communities as well as promote fungal diversity maintenance in subtropical mountain forest ecosystems.
Keywords: community composition; deterministic process; host preferences; phyllosphere; α-diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Distribution patterns of fungal community diversity in the dominant tree species Dacrydium pectinatum and Vatica mangachapoi in tropical rainforests.Microbiol Spectr. 2025 Jun 3;13(6):e0309224. doi: 10.1128/spectrum.03092-24. Epub 2025 Apr 17. Microbiol Spectr. 2025. PMID: 40243370 Free PMC article.
-
Plants Play Stronger Effects on Soil Fungal than Bacterial Communities and Co-Occurrence Network Structures in a Subtropical Tree Diversity Experiment.Microbiol Spectr. 2022 Jun 29;10(3):e0013422. doi: 10.1128/spectrum.00134-22. Epub 2022 Apr 27. Microbiol Spectr. 2022. PMID: 35475656 Free PMC article.
-
Composition and driving factors of arbuscular mycorrhizal fungal communities in the roots and rhizosphere soil of naturally regenerated Phoebe bournei seedlings in Guizhou Province, China.Microbiol Spectr. 2025 Aug 5;13(8):e0021025. doi: 10.1128/spectrum.00210-25. Epub 2025 Jul 7. Microbiol Spectr. 2025. PMID: 40621907 Free PMC article.
-
Advanced research tools for fungal diversity and its impact on forest ecosystem.Environ Sci Pollut Res Int. 2022 Jun;29(30):45044-45062. doi: 10.1007/s11356-022-20317-8. Epub 2022 Apr 23. Environ Sci Pollut Res Int. 2022. PMID: 35460003 Review.
-
Metabolic niches in the rhizosphere microbiome: dependence on soil horizons, root traits and climate variables in forest ecosystems.Front Plant Sci. 2024 Apr 5;15:1344205. doi: 10.3389/fpls.2024.1344205. eCollection 2024. Front Plant Sci. 2024. PMID: 38645395 Free PMC article. Review.
Cited by
-
Spatiotemporal dynamics reveal high turnover and contrasting assembly processes in fungal communities across contiguous habitats of tropical forests.Environ Microbiome. 2025 Feb 15;20(1):23. doi: 10.1186/s40793-025-00683-9. Environ Microbiome. 2025. PMID: 39955594 Free PMC article.
-
Distinct strategies of soil bacterial generalists and specialists in temperate deciduous broad-leaved forests.Appl Environ Microbiol. 2025 Aug 20;91(8):e0099225. doi: 10.1128/aem.00992-25. Epub 2025 Jul 30. Appl Environ Microbiol. 2025. PMID: 40736419 Free PMC article.
-
Habitat and tree species identity shape aboveground and belowground fungal communities in central European forests.Front Microbiol. 2023 Mar 6;14:1067906. doi: 10.3389/fmicb.2023.1067906. eCollection 2023. Front Microbiol. 2023. PMID: 36950169 Free PMC article.
-
Pseudophylloporus Gen. nov. and Rubroleccinum Gen. nov., Two New Genera Revealed by Morphological and Phylogenetic Evidences in the Family Boletaceae from Subtropical China.J Fungi (Basel). 2024 Nov 25;10(12):817. doi: 10.3390/jof10120817. J Fungi (Basel). 2024. PMID: 39728313 Free PMC article.
-
Distribution patterns of fungal community diversity in the dominant tree species Dacrydium pectinatum and Vatica mangachapoi in tropical rainforests.Microbiol Spectr. 2025 Jun 3;13(6):e0309224. doi: 10.1128/spectrum.03092-24. Epub 2025 Apr 17. Microbiol Spectr. 2025. PMID: 40243370 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

