Plasmid-Assisted Horizontal Transfer of a Large lsa(E)-Carrying Genomic Island in Enterococcus faecalis
- PMID: 35863017
- PMCID: PMC9430800
- DOI: 10.1128/spectrum.00154-22
Plasmid-Assisted Horizontal Transfer of a Large lsa(E)-Carrying Genomic Island in Enterococcus faecalis
Abstract
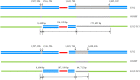
The horizontal transfer of genomic islands is essential for the adaptation and evolution of Enterococcus faecalis. In this study, three porcine E. faecalis strains, each harboring a large lsa(E)-carrying genomic island, were identified. When using the E. faecalis OG1RF as the recipient, the horizontal transfer of the lsa(E)-carrying genomic island occurred only from E. faecalis E512, which also harbored a pheromone-responsive conjugative plasmid, but not from the other two E. faecalis strains, E533 and E509, which lacked such a plasmid. Subsequently, through plasmid curing of E. faecalis E512 and plasmid introduction into E. faecalis E533, the pheromone-responsive conjugative plasmid was identified to be indispensable for the horizontal transfer of the lsa(E)-carrying genomic island and a subsequent homologous recombination between the chromosomal DNA of the donor and the recipient. In addition, the presence of a chromosomally-located conjugative transposon, Tn916, in E. faecalis E509 could not mediate the horizontal transfer of the lsa(E)-carrying genomic island, although Tn916 itself could transfer by conjugation. Thus, these data highlight the role of the pheromone-responsive conjugative plasmid in the transfer of the lsa(E)-carrying genomic island in E. faecalis, thereby establishing the dual role of pheromone-responsive conjugative plasmids in contributing to the dissemination of both plasmid-borne resistance genes and chromosomally-located genomic islands. IMPORTANCE In this study, it was shown that a pheromone-responsive conjugative plasmid played an indispensable role in the horizontal transfer of a lsa(E)-carrying genomic island. This finding indicates a dual role of the pheromone-responsive conjugative plasmid in disseminating both plasmid-borne resistance genes and chromosomally-located genomic islands. The role of the pheromone-responsive conjugative plasmid in disseminating chromosomal genomic islands is suggested to be essential in the genomic evolution of E. faecalis, which has become one of the leading nosocomial pathogens worldwide.
Keywords: Enterococcus faecalis; Tn916; conjugative plasmids; genomic island; horizontal transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sood S, Malhotra M, Das BK, Kapil A. 2008. Enterococcal infections & antimicrobial resistance. Indian J Med Res 128:111–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

