Specificity of Immunoglobulin Response to Nontuberculous Mycobacteria Infection in People with Cystic Fibrosis
- PMID: 35863022
- PMCID: PMC9430546
- DOI: 10.1128/spectrum.01874-22
Specificity of Immunoglobulin Response to Nontuberculous Mycobacteria Infection in People with Cystic Fibrosis
Abstract
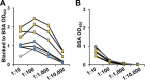
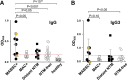
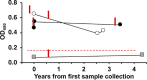
Nontuberculous mycobacteria (NTM) infections are increasingly prevalent in chronic lung diseases, including cystic fibrosis (CF). Mycobacterium abscessus is of particular concern due to relatively greater virulence and intrinsic antimicrobial resistance. Airway culture identification, the standard method for detecting pulmonary infection, is hindered by low sensitivity, long culture times, and reliance on sputum production or lavage. A culture-independent test for detecting NTM infection could complement, or replace, sputum culture, which is becoming more difficult to obtain with reduced sputum production by people with CF (pwCF) on highly effective modulator therapy. We describe an assay for the detection of plasma anti-M. abscessus antibodies of pwCF to antigens from M. abscessus lysates. Anti-M. abscessus IgG and IgA, but not IgM, discriminated with high specificity subjects infected with M. abscessus from those infected by M. avium complex, and from those with distant or no NTM infections. The IgG3 subclass predominated with minor contributions by other subclasses. Both aqueous and organic soluble antigens were recognized by plasma IgG. A validation cohort measuring IgG and IgG3 identified M. abscessus positive subjects, and elevated IgG was sustained over several years. These studies show the benefit of M. abscessus cell lysates to detect plasma IgG of subjects with CF and M. abscessus infections. Subclass analysis suggests that IgG3 is the predominant subtype in these subjects with chronic bacterial infections suggesting a defect in class maturation. Serodiagnosis could be useful to monitor M. abscessus group infections in chronic lung disease as an adjunct or alternative to culture. IMPORTANCE Lung infections with nontuberculous mycobacteria (NTM), and particularly Mycobacterium abscessus, a pathogen with high antibiotic resistance, are of great concern due to poor clinical outcomes and challenging detection in people with cystic fibrosis and other diseases. Standard detection methods are insensitive and increasingly difficult. We describe the measurement of NTM-specific antibodies from plasma to identify subjects infected with M. abscessus. The assay is sensitive and provides information on the immune response to NTM infections. This assay could be used to help identify subjects with NTM pulmonary infections and track disease progression, either alone or in conjunction with other tests.
Keywords: ELISA; cystic fibrosis; immunoglobulins; nontuberculous mycobacteria; serodiagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

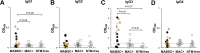
Similar articles
-
IgA Serological Response for the Diagnosis of Mycobacterium abscessus Infections in Patients with Cystic Fibrosis.Microbiol Spectr. 2022 Jun 29;10(3):e0019222. doi: 10.1128/spectrum.00192-22. Epub 2022 May 18. Microbiol Spectr. 2022. PMID: 35583329 Free PMC article.
-
Deficient Complement Opsonization Impairs Mycobacterium avium Killing by Neutrophils in Cystic Fibrosis.Microbiol Spectr. 2023 Feb 14;11(1):e0327922. doi: 10.1128/spectrum.03279-22. Epub 2023 Jan 18. Microbiol Spectr. 2023. PMID: 36651756 Free PMC article.
-
Investigating Nontuberculous Mycobacteria Transmission at the Colorado Adult Cystic Fibrosis Program.Am J Respir Crit Care Med. 2022 May 1;205(9):1064-1074. doi: 10.1164/rccm.202108-1911OC. Am J Respir Crit Care Med. 2022. PMID: 35085056 Free PMC article.
-
Nontuberculous Mycobacterial Infections in Cystic Fibrosis.Clin Chest Med. 2022 Dec;43(4):697-716. doi: 10.1016/j.ccm.2022.06.010. Clin Chest Med. 2022. PMID: 36344075 Review.
-
Pulmonary nontuberculous mycobacterial infections among women with cystic fibrosis and non-cystic fibrosis bronchiectasis.Ther Adv Respir Dis. 2025 Jan-Dec;19:17534666251323181. doi: 10.1177/17534666251323181. Epub 2025 Mar 12. Ther Adv Respir Dis. 2025. PMID: 40071337 Free PMC article. Review.
Cited by
-
Divergent host humoral innate immune response to the smooth-to-rough adaptation of Mycobacterium abscessus in chronic infection.Front Cell Infect Microbiol. 2025 Mar 18;15:1445660. doi: 10.3389/fcimb.2025.1445660. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40171164 Free PMC article.
-
Culture independent markers of nontuberculous mycobacterial (NTM) lung infection and disease in the cystic fibrosis airway.Tuberculosis (Edinb). 2023 Jan;138:102276. doi: 10.1016/j.tube.2022.102276. Epub 2022 Nov 17. Tuberculosis (Edinb). 2023. PMID: 36417800 Free PMC article.
-
Immunological and microbial shifts in the aging rhesus macaque lung during nontuberculous mycobacterial infection.mBio. 2024 Jun 12;15(6):e0082924. doi: 10.1128/mbio.00829-24. Epub 2024 May 21. mBio. 2024. PMID: 38771046 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

