Synergic Heterodinuclear Catalysts for the Ring-Opening Copolymerization (ROCOP) of Epoxides, Carbon Dioxide, and Anhydrides
- PMID: 35863044
- PMCID: PMC9350912
- DOI: 10.1021/acs.accounts.2c00197
Synergic Heterodinuclear Catalysts for the Ring-Opening Copolymerization (ROCOP) of Epoxides, Carbon Dioxide, and Anhydrides
Abstract
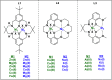
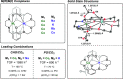
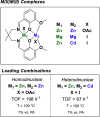
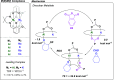
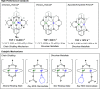
The development of sustainable plastic materials is an essential target of chemistry in the 21st century. Key objectives toward this goal include utilizing sustainable monomers and the development of polymers that can be chemically recycled/degraded. Polycarbonates synthesized from the ring-opening copolymerization (ROCOP) of epoxides and CO2, and polyesters synthesized from the ROCOP of epoxides and anhydrides, meet these criteria. Despite this, designing efficient catalysts for these processes remains challenging. Typical issues include the requirement for high catalyst loading; low catalytic activities in comparison with other commercialized polymerizations; and the requirement of costly, toxic cocatalysts. The development of efficient catalysts for both types of ROCOP is highly desirable. This Account details our work on the development of catalysts for these two related polymerizations and, in particular, focuses on dinuclear complexes, which are typically applied without any cocatalyst. We have developed mechanistic hypotheses in tandem with our catalysts, and throughout the Account, we describe the kinetic, computational, and structure-activity studies that underpin the performance of these catalysts. Our initial research on homodinuclear M(II)M(II) complexes for cyclohexene oxide (CHO)/CO2 ROCOP provided data to support a chain shuttling catalytic mechanism, which implied different roles for the two metals in the catalysis. This mechanistic hypothesis inspired the development of mixed-metal, heterodinuclear catalysts. The first of this class of catalysts was a heterodinuclear Zn(II)Mg(II) complex, which showed higher rates than either of the homodinuclear [Zn(II)Zn(II) and Mg(II)Mg(II)] analogues for CHO/CO2 ROCOP. Expanding on this finding, we subsequently developed a Co(II)Mg(II) complex that showed field leading rates for CHO/CO2 ROCOP and allowed for unique insight into the role of the two metals in this complex, where it was established that the Mg(II) center reduced transition state entropy and the Co(II) center reduced transition state enthalpy. Following these discoveries, we subsequently developed a range of heterodinuclear M(III)M(I) catalysts that were capable of catalyzing a broad range of copolymerizations, including the ring-opening copolymerization of CHO/CO2, propylene oxide (PO)/CO2, and CHO/phthalic anhydride (PA). Catalysts featuring Co(III)K(I) and Al(III)K(I) were found to be exceptionally effective for PO/CO2 and CHO/PA ROCOP, respectively. Such M(III)M(I) complexes operate through a dinuclear metalate mechanism, where the M(III) binds and activates monomers while the M(I) species binds the polymer change in close proximity to allow for insertion into the activated monomer. Our research illustrates how careful catalyst design can yield highly efficient systems and how the development of mechanistic understanding aids this process. Avenues of future research are also discussed, including the applicability of these heterodinuclear catalysts in the synthesis of sustainable materials.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.K.W. is a director of Econic Technologies.
Figures



References
-
- Diment W. T.; Gregory G. L.; Kerr R. W. F.; Phanopoulos A.; Buchard A.; Williams C. K. Catalytic Synergy Using Al(III) and Group 1 Metals to Accelerate Epoxide and Anhydride Ring-Opening Copolymerizations. ACS Catal. 2021, 11, 12532–12542. 10.1021/acscatal.1c04020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

