Cervical cell lift: A novel triage method for the spatial mapping and grading of precancerous cervical lesions
- PMID: 35863292
- PMCID: PMC9301573
- DOI: 10.1016/j.ebiom.2022.104157
Cervical cell lift: A novel triage method for the spatial mapping and grading of precancerous cervical lesions
Abstract
Background: Primary HPV screening, due to its low specificity, requires an additional liquid-based cytology (LBC) triage test. However, even with LBC triage there has been a near doubling in the number of patients referred for colposcopy in recent years, the majority having low-grade disease.
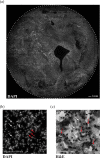
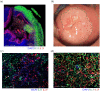
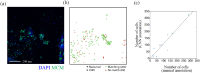
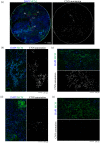
Methods: To counter this, a triage test that generates a spatial map of the cervical surface at a molecular level has been developed which removes the subjectivity associated with LBC by facilitating identification of lesions in their entirety. 50 patients attending colposcopy were recruited to participate in a pilot study to evaluate the test. For each patient, cells were lifted from the cervix onto a membrane (cervical cell lift, CCL) and immunostained with a biomarker of precancerous cells, generating molecular maps of the cervical surface. These maps were analysed to detect high-grade lesions, and the results compared to the final histological diagnosis.
Findings: We demonstrated that spatial molecular mapping of the cervix has a sensitivity of 90% (95% CI 69-98) (positive predictive value 81% (95% CI 60-92)) for the detection of high-grade disease, and that AI-based analysis could aid disease detection through automated flagging of biomarker-positive cells.
Interpretation: Spatial molecular mapping of the CCL improved the rate of detection of high-grade disease in comparison to LBC, suggesting that this method has the potential to decisively identify patients with clinically relevant disease that requires excisional treatment.
Funding: CRUK Early Detection Project award, Jordan-Singer BSCCP award, Addenbrooke's Charitable Trust, UK-MRC, Janssen Pharmaceuticals/Advanced Sterilisation Products, and NWO.
Keywords: Biomarker for cervical screening; CIN; Cervical cancer; Cytology; HPV; Methodology for cervical screening; Non-invasive sampling; Spatial mapping of lesion; Triage test.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of interests Dr Egawa, Dr Shiraz and Prof Doorbar report grants from Janssen Pharmaceuticals/Advanced Sterilization Products (ASP). Dr Egawa was working for Maruho Co., Ltd as a consultant outside of this work. Ms Nicholas, Ms Romashova, Dr Griffin, Prof Sasieni and Dr Crawford have nothing to disclose pertaining to this project.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet North Am Ed. 2014;383:524–532. - PubMed
-
- Simms KT, Hanley SJB, Smith MA, Keane A, Canfell K. Articles impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5:e223–e234. - PubMed
-
- Arbyn M, Roelens J, Cuschieri K, et al. The APTIMA HPV assay versus the hybrid capture 2 test in triage of women with ASC-US or LSIL cervical cytology: a meta-analysis of the diagnostic accuracy. Int J Cancer. 2013;132:101–108. - PubMed

