Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: a systematic review and meta-analysis
- PMID: 35863630
- PMCID: PMC9293401
- DOI: 10.1016/j.cmi.2022.07.008
Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: a systematic review and meta-analysis
Abstract
Background: Randomized controlled trials (RCT) established the mortality reduction by tocilizumab (Actemra), baricitinib (Olumiant), and sarilumab (Kevzara) in hospitalized COVID-19 patients. However, uncertainty remains about which treatment performs best in patients receiving corticosteroids.
Objectives: To estimate probabilities of noninferiority between baricitinib and sarilumab compared to tocilizumab in patients treated with corticosteroids.
Data sources: PubMed, Embase, Cochrane Library, and MedRxiv.
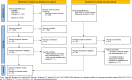
Study eligibility criteria: Eligible RCTs assigning hospitalized adults with COVID-19 treated with corticosteroids to tocilizumab or baricitinib or sarilumab versus standard of care or placebo (control).
Methods: Reviewers independently abstracted published data and assessed study quality with the Risk of Bias 2 tool. Unpublished data, if required, were requested from authors of included studies. The outcome of interest was all-cause mortality at 28 days.
Participants: Twenty-seven RCTs with 13 549 patients were included. Overall, the risk of bias was low. Bayesian pairwise meta-analyses were used to aggregate results of each treatment versus control. The average odds ratio for mortality was 0.78 (95% credible interval [CrI]: 0.65, 0.94) for tocilizumab; 0.78 (95% CrI: 0.56, 1.03) for baricitinib; and 0.91 (95% CrI: 0.60, 1.40) for sarilumab. The certainty of evidence (GRADE) ranged from moderate to low. Bayesian meta-regressions with multiple priors were used to estimate probabilities of noninferiority (margin of 13% greater effect by tocilizumab). Compared to tocilizumab, there were ≤94% and 90% probabilities of noninferiority with baricitinib and sarilumab, respectively.
Results: All but two studies included data with only indirect evidence for the comparison of interest.
Conclusions: Among hospitalized COVID-19 treated with corticosteroids, there are high probabilities that both baricitinib and sarilumab are associated with similar mortality reductions in comparison to tocilizumab.
Keywords: Baricitinib; COVID-19; Corticosteroids; Mortality; Sarilumab; Tocilizumab.
Copyright © 2022 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Successful immunomodulators for the treatment of COVID-19 have opened the pathway for comparative trials.Clin Microbiol Infect. 2023 Jan;29(1):7-9. doi: 10.1016/j.cmi.2022.10.016. Epub 2022 Oct 17. Clin Microbiol Infect. 2023. PMID: 36257548 Free PMC article. No abstract available.
References
-
- Lee T.C., Murthy S., Corpo O.D., Senécal J., Butler-Laporte G., Sohani Z.N., et al. Remdesivir for the treatment of COVID-19: an updated systematic review and meta-analysis [e-pub ahead of print] Clin Microbiol Infect. 2022 http://10.1016/j.cmi.2022.04.018 [cited 05-18-2022] - DOI - PMC - PubMed
-
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., et al. The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

