Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities
- PMID: 35863823
- PMCID: PMC9310257
- DOI: 10.1136/jitc-2022-004892
Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities
Abstract
Background: Immune microenvironment is well recognized as a critical regulator across cancer types, despite its complex roles in different disease conditions. Intrahepatic cholangiocarcinoma (iCCA) is characterized by a tumor-reactive milieu, emphasizing a deep insight into its immunogenomic profile to provide prognostic and therapeutic implications.
Methods: We performed genomic, transcriptomic, and proteomic characterization of 255 paired iCCA and adjacent liver tissues. We validated our findings through H&E staining (n=177), multiplex immunostaining (n=188), single-cell RNA sequencing (scRNA-seq) (n=10), in vitro functional studies, and in vivo transposon-based mouse models.
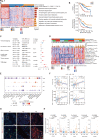
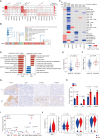
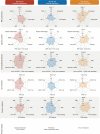
Results: Integrated multimodule data identified three immune subgroups with distinct clinical, genetic, and molecular features, designated as IG1 (immune-suppressive, 25.1%), IG2 (immune-exclusion, 42.7%), and IG3 (immune-activated, 32.2%). IG1 was characterized by excessive infiltration of neutrophils and immature dendritic cells (DCs). The hallmark of IG2 was the relatively higher tumor-proliferative activity and tumor purity. IG3 exhibited an enrichment of adaptive immune cells, natural killer cells, and activated DCs. These immune subgroups were significantly associated with prognosis and validated in two independent cohorts. Tumors with KRAS mutations were enriched in IG1 and associated with myeloid inflammation-dominated immunosuppression. Although tumor mutation burden was relatively higher in IG2, loss of heterozygosity in human leucocyte antigen and defects in antigen presentation undermined the recognition of neoantigens, contributing to immune-exclusion behavior. Pathological analysis confirmed that tumor-infiltrating lymphocytes and tertiary lymphoid structures were both predominant in IG3. Hepatitis B virus (HBV)-related samples tended to be under-represented in IG1, and scRNA-seq analyses implied that HBV infection indeed alleviated myeloid inflammation and reinvigorated antitumor immunity.
Conclusions: Our study elucidates that the immunogenomic traits of iCCA are intrinsically heterogeneous among patients, posing great challenge and opportunity for the application of personalized immunotherapy.
Keywords: cytotoxicity, immunologic; drug therapy, combination; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Shi G-M, Jian Z, Fan J, et al. . Phase II study of lenvatinib in combination with GEMOX chemotherapy for advanced intrahepatic cholangiocarcinoma. Journal of Clinical Oncology 2021;39:e16163. 10.1200/JCO.2021.39.15_suppl.e16163 - DOI
-
- Imfinzi plus chemotherapy significantly improved overall survival in 1st-line advanced biliary tract cancer in TOPAZ-1 phase III trial at interim analysis, 2021. Available: https://www.astrazeneca.com/content/astraz/media-centre/pressreleases/20...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
