Impact of clinical domains other than arthritis on composite outcomes in psoriatic arthritis: comparison of treatment effects in the SEAM-PsA trial
- PMID: 35863864
- PMCID: PMC9310247
- DOI: 10.1136/rmdopen-2022-002366
Impact of clinical domains other than arthritis on composite outcomes in psoriatic arthritis: comparison of treatment effects in the SEAM-PsA trial
Abstract
Objective: We used the Study of Etanercept And Methotrexate in Combination or as Monotherapy in Subjects with Psoriatic Arthritis (SEAM-PsA) data set to examine the impact of presence of enthesitis, dactylitis, nail disease and/or psoriasis on treatment response in patients with early psoriatic arthritis (PsA).
Methods: This post hoc analysis evaluated the effect of baseline Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index (EI), Leeds Enthesitis Index (LEI), Leeds Dactylitis Index (LDI), modified Nail Psoriasis Severity Index (mNAPSI) scores and body surface area (BSA) on composite outcomes of minimal disease activity (MDA) responses, Psoriatic Arthritis Disease Activity Score (PASDAS) low disease activity (LDA), PASDAS changes and Good Responses and Disease Activity Index for Psoriatic Arthritis (DAPSA) scores at Week 24.
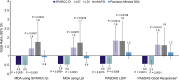
Results: Overall, 851 patients completed the SEAM-PsA trial and were included in the analysis. Baseline enthesitis (SPARCC EI>0 vs SPARCC EI=0 or LEI>0 vs LEI=0) was not associated with improved outcomes. Baseline dactylitis (LDI>0 vs LDI=0) was positively associated with improved MDA (OR: 1.4, p=0.0457), PASDAS LDA (OR: 1.8, p=0.0014) and Good Responses (OR: 1.6, p=0.0101) and greater reductions in PASDAS (estimate: -0.9, p<0.0001) and DAPSA scores (estimate: -3.8, p=0.0155) at Week 24. Similarly, baseline nail disease (mNAPSI >1 vs mNAPSI≤1) was positively associated with improved MDA (OR: 1.8, p=0.0233) and PASDAS LDA (OR: 1.8, p=0.0168) responses and greater reduction in PASDAS (estimate: -0.7, p=0.0005) at Week 24.
Conclusions: Results from our analysis suggest that presence of dactylitis and nail disease, but not enthesitis, are associated with improved outcomes in patients with early PsA who were treated with methotrexate and/or etanercept.
Keywords: Antirheumatic Agents; Arthritis; Methotrexate; Tumor Necrosis Factor Inhibitors.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PSH has received grant/research support and/or speaker/honoraria from AbbVie, Novartis, Janssen and Pfizer. PJM has received research grants and has served as a consultant or has been a speaker for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN Pharma and UCB. AK declares no conflicts for this work. LCC has received grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis and Pfizer; has worked as a paid consultant for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, Novartis, Pfizer and UCB; and has been paid as a speaker for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, medac, Novartis, Pfizer and UCB. AO has received research grants from AbbVie, Novartis and Pfizer to University of Pennsylvania and Amgen to FORWARD/NDB; has received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Gilead, Janssen, Lilly, Novartis, Pfizer and UCB; and reports receipt of royalties from Novartis (to spouse). AD has received research grants from AbbVie, GlaxoSmithKline, Novartis, Pfizer and UCB and has received consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer and UCB. VS has received consulting fees from AbbVie, Amgen, Arena, Aria, AstraZeneca, Bayer, Bioventus, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Corrona, Crescendo/Myriad, EMD Serono, Equillium, Flexion, Genentech/Roche, Glenmark, GSK, Horizon, Inmedix, Janssen, Kypha, Eli Lilly, Merck, Novartis, Pfizer, Regeneron, Samsung, Sandoz, Sanofi, Servier, Setpoint and UCB. GK is an employee of and owns stock in Amgen. DC is an employee of and owns stock in Amgen. LXHL is an employee of and owns stock in Amgen. DDG has received research grants from Amgen, AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB and has received consulting fees from Amgen, AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, Novartis, Pfizer and UCB.
Figures

Similar articles
-
Efficacy of tofacitinib on enthesitis in patients with active psoriatic arthritis: analysis of pooled data from two phase 3 studies.Arthritis Res Ther. 2023 Aug 22;25(1):153. doi: 10.1186/s13075-023-03108-5. Arthritis Res Ther. 2023. PMID: 37608391 Free PMC article. Clinical Trial.
-
Performance of composite measures used in a trial of etanercept and methotrexate as monotherapy or in combination in psoriatic arthritis.Rheumatology (Oxford). 2021 Mar 2;60(3):1137-1147. doi: 10.1093/rheumatology/keaa271. Rheumatology (Oxford). 2021. PMID: 32864685 Free PMC article. Clinical Trial.
-
Potential Impact of Sex and BMI on Response to Therapy in Psoriatic Arthritis: Post Hoc Analysis of Results From the SEAM-PsA Trial.J Rheumatol. 2022 Aug;49(8):885-893. doi: 10.3899/jrheum.211037. Epub 2022 Apr 15. J Rheumatol. 2022. PMID: 35428718 Clinical Trial.
-
Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI).Arthritis Care Res (Hoboken). 2011 Nov;63 Suppl 11:S64-85. doi: 10.1002/acr.20577. Arthritis Care Res (Hoboken). 2011. PMID: 22588772 Review. No abstract available.
-
Clinical enthesitis in patient with psoriatic arthritis, a systematic literature review with metaanalysis.Joint Bone Spine. 2025 Mar;92(2):105807. doi: 10.1016/j.jbspin.2024.105807. Epub 2024 Oct 30. Joint Bone Spine. 2025. PMID: 39486614
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
