A Fibrinogen-Mimicking, Activated-Platelet-Sensitive Nanocoacervate Enhances Thrombus Targeting and Penetration of Tissue Plasminogen Activator for Effective Thrombolytic Therapy
- PMID: 35864062
- PMCID: PMC11468879
- DOI: 10.1002/adhm.202201265
A Fibrinogen-Mimicking, Activated-Platelet-Sensitive Nanocoacervate Enhances Thrombus Targeting and Penetration of Tissue Plasminogen Activator for Effective Thrombolytic Therapy
Abstract
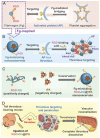
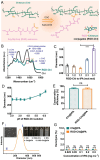
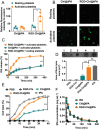
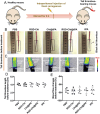
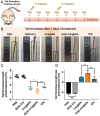
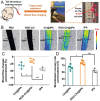
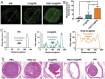
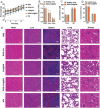
The development of a fibrinolytic system with long circulation time, high thrombus targeting, efficient thrombus penetration, effective thrombolysis, and minimal hemorrhagic risk remains a major challenge. Herein, inspired by fibrinogen binding to activated platelets in thrombosis, this article reports a fibrinogen-mimicking, activated-platelet-sensitive nanocoacervate to enhance thrombus penetration of tissue plasminogen activator (tPA) for targeted thrombolytic therapy. This biomimetic nanothrombolytic system, denoted as RGD-Chi@tPA, is constructed by "one-pot" coacervation through electrostatic interactions between positively charged arginine-glycine-aspartic acid (RGD)-grafted chitosan (RGD-Chi) and negatively charged tPA. Flow cytometry and confocal laser scanning microscopy measurements show targeting of RGD-Chi@tPA to activated platelets. Controlled tPA release triggered by activated platelets at a thrombus site is demonstrated. Its targeted fibrinolytic and thrombolytic activities are measured in in vitro models. The pharmacokinetic profiles show that RGD-Chi@tPA can significantly prolong circulation time compared to free tPA. In a mouse tail thrombus model, RGD-Chi@tPA displays efficient thrombus targeting and penetration, enabling a complete vascular recanalization as confirmed by the fluorescence imaging, histochemical assay, and laser speckle contrast imager. Consequently, RGD-Chi@tPA induces a substantial enhancement in thrombolysis with minimal hemorrhagic risk compared to free tPA. This simple, effective, and safe platform holds great promise for the development of thrombolytic nanomedicines.
Keywords: chitosan; fibrinogen-mimicking nanocoacervate; targeted thrombolysis; thrombus penetration; tissue plasminogen activators.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Collen D., Lijnen H. R., Blood 1991, 78, 3114. - PubMed
-
- Raskob G. E., Angcahisuksiri P., Blanco A. N., Buller H., Gallus A., Hunt B. J., Hylek E. M., Kakkar A., Kostantinides S. V., McCumber M., Ozaki Y., Wendelboe A., Weitz J. I., Thromb. Res. 2014, 134, 931. - PubMed
-
- Marder V., Blood Rev. 2001, 15, 143. - PubMed
-
- Bode C., Runge M. S., Haber E., Clin. Cardiol. 1990, 13, 375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

