Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma
- PMID: 35864093
- PMCID: PMC9304377
- DOI: 10.1038/s41467-022-31799-y
Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma
Abstract
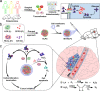
Glioblastoma multiforme (GBM) is an aggressive brain cancer with a poor prognosis and few treatment options. Here, building on the observation of elevated lactate (LA) in resected GBM, we develop biomimetic therapeutic nanoparticles (NPs) that deliver agents for LA metabolism-based synergistic therapy. Because our self-assembling NPs are encapsulated in membranes derived from glioma cells, they readily penetrate the blood-brain barrier and target GBM through homotypic recognition. After reaching the tumors, lactate oxidase in the NPs converts LA into pyruvic acid (PA) and hydrogen peroxide (H2O2). The PA inhibits cancer cell growth by blocking histones expression and inducing cell-cycle arrest. In parallel, the H2O2 reacts with the delivered bis[2,4,5-trichloro-6-(pentyloxycarbonyl)phenyl] oxalate to release energy, which is used by the co-delivered photosensitizer chlorin e6 for the generation of cytotoxic singlet oxygen to kill glioma cells. Such a synergism ensures strong therapeutic effects against both glioma cell-line derived and patient-derived xenograft models.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Comment in
-
New local therapy strategies for brain tumours.Nat Rev Neurol. 2022 Sep;18(9):509. doi: 10.1038/s41582-022-00710-0. Nat Rev Neurol. 2022. PMID: 35945457 No abstract available.
References
-
- Metwali H, Valenca MM. Intraoperative assistive technologies and extent of resection in glioma surgery: a systematic review of prospective controlled studies. Neurosurg. Rev. 2015;38:226–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

