Inhibition of pancreatic EZH2 restores progenitor insulin in T1D donor
- PMID: 35864094
- PMCID: PMC9304326
- DOI: 10.1038/s41392-022-01034-7
Inhibition of pancreatic EZH2 restores progenitor insulin in T1D donor
Abstract
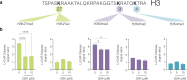
Type 1 diabetes (T1D) is an autoimmune disease that selectively destroys insulin-producing β-cells in the pancreas. An unmet need in diabetes management, current therapy is focussed on transplantation. While the reprogramming of progenitor cells into functional insulin-producing β-cells has also been proposed this remains controversial and poorly understood. The challenge is determining why default transcriptional suppression is refractory to exocrine reactivation. After the death of a 13-year-old girl with established insulin-dependent T1D, pancreatic cells were harvested in an effort to restore and understand exocrine competence. The pancreas showed classic silencing of β-cell progenitor genes with barely detectable insulin (Ins) transcript. GSK126, a highly selective inhibitor of EZH2 methyltransferase activity influenced H3K27me3 chromatin content and transcriptional control resulting in the expression of core β-cell markers and ductal progenitor genes. GSK126 also reinstated Ins gene expression despite absolute β-cell destruction. These studies show the refractory nature of chromatin characterises exocrine suppression influencing β-cell plasticity. Additional regeneration studies are warranted to determine if the approach of this n-of-1 study generalises to a broader T1D population.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

