A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma
- PMID: 35864115
- PMCID: PMC9304402
- DOI: 10.1038/s41467-022-31262-y
A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma
Abstract
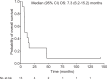
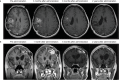
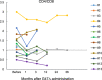
Here, we report the results of a phase I/II, single-arm study (UMIN-CTR Clinical Trial Registry UMIN000002661) assessing the safety (primary endpoint) of G47∆, a triple-mutated oncolytic herpes simplex virus type 1, in Japanese adults with recurrent/progressive glioblastoma despite radiation and temozolomide therapies. G47Δ was administered intratumorally at 3 × 108 pfu (low dose) or 1 × 109 pfu (set dose), twice to identical coordinates within 5-14 days. Thirteen patients completed treatment (low dose, n = 3; set dose, n = 10). Adverse events occurred in 12/13 patients. The most common G47Δ-related adverse events were fever, headache and vomiting. Secondary endpoint was the efficacy. Median overall survival was 7.3 (95%CI 6.2-15.2) months and the 1-year survival rate was 38.5%, both from the last G47∆ administration. Median progression-free survival was 8 (95%CI 7-34) days from the last G47∆ administration, mainly due to immediate enlargement of the contrast-enhanced area of the target lesion on MRI. Three patients survived >46 months. One complete response (low dose) and one partial response (set dose) were seen at 2 years. Based on biopsies, post-administration MRI features (injection site contrast-enhancement clearing and entire tumor enlargement) likely reflected tumor cell destruction via viral replication and lymphocyte infiltration towards tumor cells, the latter suggesting the mechanism for "immunoprogression" characteristic to this therapy. This study shows that G47Δ is safe for treating recurrent/progressive glioblastoma and warrants further clinical development.
© 2022. The Author(s).
Conflict of interest statement
T.T. owns the patent right for G47∆ in Japan (“Viral vectors and their use in therapeutic methods.” 4212897). All other authors have no conflict of interest to declare.
Figures



References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

