A quantum chemical molecular dynamics repository of solvated ions
- PMID: 35864118
- PMCID: PMC9304403
- DOI: 10.1038/s41597-022-01527-8
A quantum chemical molecular dynamics repository of solvated ions
Abstract
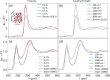
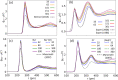
The importance of ion-solvent interactions in predicting specific ion effects in contexts ranging from viral activity through to electrolyte viscosity cannot be underestimated. Moreover, investigations of specific ion effects in nonaqueous systems, highly relevant to battery technologies, biochemical systems and colloid science, are severely limited by data deficiency. Here, we report IonSolvR - a collection of more than 3,000 distinct nanosecond-scale ab initio molecular dynamics simulations of ions in aqueous and non-aqueous solvent environments at varying effective concentrations. Density functional tight binding (DFTB) is used to detail the solvation structure of up to 55 solutes in 28 different protic and aprotic solvents. DFTB is a fast quantum chemical method, and as such enables us to bridge the gap between efficient computational scaling and maintaining accuracy, while using an internally-consistent simulation technique. We validate the database against experimental data and provide guidance for accessing individual IonSolvR records.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Ionic hydrogen-bond networks and ion solvation. 1. An efficient Monte Carlo/quantum mechanical method for structural search and energy computations: ammonium/water.J Phys Chem A. 2009 Mar 26;113(12):2967-74. doi: 10.1021/jp808486k. J Phys Chem A. 2009. PMID: 19243164
-
Solvation and Ion-Pairing Effects of Choline Acetate Electrolyte in Protic and Aprotic Solvents Studied by NMR Titrations.Chemphyschem. 2022 Jan 5;23(1):e202100602. doi: 10.1002/cphc.202100602. Epub 2021 Nov 3. Chemphyschem. 2022. PMID: 34708481 Free PMC article.
-
Modeling infrared and vibrational circular dichroism spectra of complex systems: the DFTB/fluctuating charges route.Phys Chem Chem Phys. 2025 May 28;27(21):11198-11209. doi: 10.1039/d5cp00228a. Phys Chem Chem Phys. 2025. PMID: 40377067
-
High-Throughput Aqueous Electrolyte Structure Prediction Using IonSolvR and Equivariant Graph Neural Network Potentials.J Phys Chem Lett. 2023 Oct 26;14(42):9508-9515. doi: 10.1021/acs.jpclett.3c01783. Epub 2023 Oct 16. J Phys Chem Lett. 2023. PMID: 37845640
-
Development of Divide-and-Conquer Density-Functional Tight-Binding Method for Theoretical Research on Li-Ion Battery.Chem Rec. 2019 Apr;19(4):746-757. doi: 10.1002/tcr.201800141. Epub 2018 Nov 21. Chem Rec. 2019. PMID: 30462370 Review.
Cited by
-
Treating Semiempirical Hamiltonians as Flexible Machine Learning Models Yields Accurate and Interpretable Results.J Chem Theory Comput. 2023 Sep 26;19(18):6185-6196. doi: 10.1021/acs.jctc.3c00491. Epub 2023 Sep 13. J Chem Theory Comput. 2023. PMID: 37705220 Free PMC article.
-
The Potential of Neural Network Potentials.ACS Phys Chem Au. 2024 Mar 21;4(3):232-241. doi: 10.1021/acsphyschemau.4c00004. eCollection 2024 May 22. ACS Phys Chem Au. 2024. PMID: 38800721 Free PMC article. Review.
-
Network pharmacology and AI in cancer research uncovering biomarkers and therapeutic targets for RALGDS mutations.Sci Rep. 2025 Mar 29;15(1):10938. doi: 10.1038/s41598-025-91568-x. Sci Rep. 2025. PMID: 40157967 Free PMC article.
References
-
- Kunz W, Henle J, Ninham BW. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004;9:19–37. doi: 10.1016/j.cocis.2004.05.005. - DOI
-
- Mazzini V, Craig VSJ. Specific-ion effects in non-aqueous systems. Curr. Opin. Colloid Interface Sci. 2016;23:82–93. doi: 10.1016/j.cocis.2016.06.009. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

