A general design of caging-group-free photoactivatable fluorophores for live-cell nanoscopy
- PMID: 35864152
- PMCID: PMC9417988
- DOI: 10.1038/s41557-022-00995-0
A general design of caging-group-free photoactivatable fluorophores for live-cell nanoscopy
Abstract
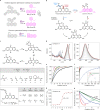
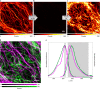
The controlled switching of fluorophores between non-fluorescent and fluorescent states is central to every super-resolution fluorescence microscopy (nanoscopy) technique, and the exploration of radically new switching mechanisms remains critical to boosting the performance of established, as well as emerging super-resolution methods. Photoactivatable dyes offer substantial improvements to many of these techniques, but often rely on photolabile protecting groups that limit their applications. Here we describe a general method to transform 3,6-diaminoxanthones into caging-group-free photoactivatable fluorophores. These photoactivatable xanthones (PaX) assemble rapidly and cleanly into highly fluorescent, photo- and chemically stable pyronine dyes upon irradiation with light. The strategy is extendable to carbon- and silicon-bridged xanthone analogues, yielding a family of photoactivatable labels spanning much of the visible spectrum. Our results demonstrate the versatility and utility of PaX dyes in fixed and live-cell labelling for conventional microscopy, as well as the coordinate-stochastic and deterministic nanoscopies STED, PALM and MINFLUX.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): R.L., M.L.B. and A.N.B. are co-inventors of a patent application (International Patent Application No. PCT/EP2021/069804) covering the photoactivatable dyes of this work, filed by the Max Planck Society. S.W.H. owns shares of Abberior GmbH and Abberior Instruments GmbH, whose dyes and MINFLUX microscope, respectively, have been used in this study. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

