Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial
- PMID: 35864254
- PMCID: PMC9388376
- DOI: 10.1038/s41591-022-01897-x
Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial
Erratum in
-
Author Correction: Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial.Nat Med. 2025 Apr;31(4):1365. doi: 10.1038/s41591-025-03619-5. Nat Med. 2025. PMID: 40102594 Free PMC article. No abstract available.
Abstract
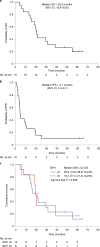
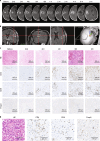
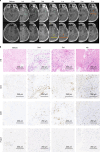
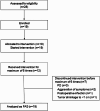
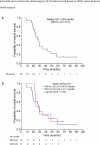
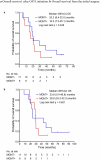
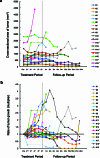
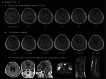
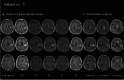
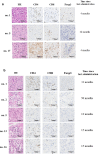
This investigator-initiated, phase 2, single-arm trial primarily assessed the efficacy of G47∆, a triple-mutated, third-generation oncolytic herpes simplex virus type 1, in 19 adult patients with residual or recurrent, supratentorial glioblastoma after radiation therapy and temozolomide (UMIN-CTR Clinical Trial Registry UMIN000015995). G47Δ was administered intratumorally and repeatedly for up to six doses. The primary endpoint of 1-yr survival rate after G47∆ initiation was 84.2% (95% confidence interval, 60.4-96.6; 16 of 19). The prespecified endpoint was met and the trial was terminated early. Regarding secondary endpoints, the median overall survival was 20.2 (16.8-23.6) months after G47∆ initiation and 28.8 (20.1-37.5) months from the initial surgery. The most common G47∆-related adverse event was fever (17 of 19) followed by vomiting, nausea, lymphocytopenia and leukopenia. On magnetic resonance imaging, enlargement of and contrast-enhancement clearing within the target lesion repeatedly occurred after each G47∆ administration, which was characteristic to this therapy. Thus, the best overall response in 2 yr was partial response in one patient and stable disease in 18 patients. Biopsies revealed increasing numbers of tumor-infiltrating CD4+/CD8+ lymphocytes and persistent low numbers of Foxp3+ cells. This study showed a survival benefit and good safety profile, which led to the approval of G47∆ as the first oncolytic virus product in Japan.
© 2022. The Author(s).
Conflict of interest statement
T.T. owns the patent right for G47∆ in Japan. All other authors have no conflict of interest to declare.
Figures

Comment in
-
Treat and repeat: oncolytic virus therapy for brain cancer.Nat Med. 2022 Aug;28(8):1540-1542. doi: 10.1038/s41591-022-01901-4. Nat Med. 2022. PMID: 35864255 No abstract available.
-
Promising OS with oncolytic virotherapy.Nat Rev Clin Oncol. 2022 Oct;19(10):616. doi: 10.1038/s41571-022-00678-2. Nat Rev Clin Oncol. 2022. PMID: 35971000 No abstract available.
References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

