Ipilimumab and nivolumab in advanced hepatocellular carcinoma after failure of prior immune checkpoint inhibitor-based combination therapies: a multicenter retrospective study
- PMID: 35864269
- PMCID: PMC10314839
- DOI: 10.1007/s00432-022-04206-8
Ipilimumab and nivolumab in advanced hepatocellular carcinoma after failure of prior immune checkpoint inhibitor-based combination therapies: a multicenter retrospective study
Abstract
Introduction: Immune checkpoint inhibitor (ICI)-based regimens are transforming the landscape of hepatocellular carcinoma (HCC) treatment. We describe the effect of combined ipilimumab and nivolumab in patients with advanced HCC after the failure of prior ICI-based combination treatments.
Methods: The clinical course of patients with advanced HCC who received combined ipilimumab and nivolumab after prior ICI-based combination therapies was assessed. Progression-free survival (PFS), overall response rate (ORR) and disease control rate (DCR) per RECIST v1.1 and mRECIST, overall survival (OS), and safety were analyzed.
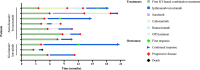
Results: Of 109 patients treated with atezolizumab and bevacizumab or other ICI-based combination treatments, ten patients received subsequent therapy with ipilimumab and nivolumab. The majority of patients had Barcelona Clinic Liver Cancer (BCLC) Stage C (80%) HCC and a preserved liver function as defined by Child-Pugh A (80%). At a median follow-up of 15.3 months, ORR for ipilimumab and nivolumab was 30% with a DCR of 40%. Median PFS was 2.9 months and the median OS was 7.4 months.
Conclusion: This retrospective study demonstrates that combined ipilimumab and nivolumab can be effective and tolerable after prior ICI-based combination therapies and provides a rationale for the prospective clinical evaluation of this treatment sequencing.
Keywords: Atezolizumab; Bevacizumab; Hepatocellular carcinoma; Immune checkpoint inhibitors; Ipilimumab; Nivolumab.
© 2022. The Author(s).
Conflict of interest statement
DR advises for Bayer and advises and has received grants from Ipsen. SM received a research grant from Ipsen. MS reports lecture honorarium from Siemens, Cook medical, Boston Scientific, LIAM, Bayer, Sirtex and research grants from Sirtex and Bayer. FPR has received honoraria for lectures and travel support from the Falk Foundation, Gilead and Novartis. AG has received honoraria for lectures, teaching, advisory activities and travel support from AbbVie, Alexion, Bayer, BMS, CSL Behring, Eisai, Gilead, Intercept, Falk, Ipsen, MSD, Merz, Novartis, Pfizer, Roche, Sanofi-Aventis, Sequana and has received research support from Intercept und Falk (NAFLD CSG) and Novartis. EDT has served as a paid consultant for AstraZeneca, Bayer, BMS, EISAI, Eli Lilly & Co, Pfizer, IPSEN, and Roche. He has received reimbursement of meeting attendance fees and travel expenses from Arqule, Astrazeneca, BMS, Bayer, Celsion and Roche, and lecture honoraria from BMS and Falk. He has received third-party funding for scientific research from Arqule, AstraZeneca, BMS, Bayer, Eli Lilly, and Roche. LSJ has received speaker honoraria from Boston. NBK has received reimbursement of meeting attendance fees and travel expenses from EISAI and lecture honorarium from Falk. All the other authors have no conflicts of interest to declare
Figures


References
-
- Abou-Alfa K, Lau G, Kudo M, Chan Stephen L, Kelley Robin K, Furuse J, Sukeepaisarnjaroen W, Kang Y-K, Van Dao T, De Toni Enrico N, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam Vincent C, Mody K, Thungappa Satheesh C, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng A-L, Qin S, Galle Peter R, Ali S, Marcovitz M, Makowsky M, He P, Kurland John F, Negro A, Sangro B (2022) Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 0(0):EVIDoa2100070 - PubMed
-
- Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, Cicin I, Merle P, Chen Y, Park J-W, Blanc J-F, Bolondi L, Klümpen H-J, Chan SL, Zagonel V, Pressiani T, Ryu M-H, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379(1):54–63 - PMC - PubMed
-
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66 - PubMed
-
- Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS (2021) Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76(4):862–873 - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

