Safety and efficacy of irinotecan, oxaliplatin, and capecitabine (XELOXIRI) regimen with or without targeted drugs in patients with metastatic colorectal cancer: a retrospective cohort study
- PMID: 35864467
- PMCID: PMC9306070
- DOI: 10.1186/s12885-022-09889-3
Safety and efficacy of irinotecan, oxaliplatin, and capecitabine (XELOXIRI) regimen with or without targeted drugs in patients with metastatic colorectal cancer: a retrospective cohort study
Abstract
Background: Five-fluorouracil, folinic acid, oxaliplatin and irinotecan (FOLFOXIRI) regimen is used as the first-line treatment for metastatic colorectal cancer (mCRC). The use of capecitabine, an oral fluoropyrimidine pro-drug, is feasible and safe; hence, it provides an interesting alternative to 5-fluorouracil in the abovementioned regimen. This study aimed to evaluate the efficacy and safety of capecitabine, oxaliplatin, and irinotecan (XELOXIRI) regimen use with or without targeted drugs in Chinese patients with mCRC.
Methods: We conducted a retrospective, longitudinal cohort study of patients with mCRC who received XELOXIRI regimen with or without targeted drugs (bevacizumab or cetuximab) every 2 weeks between January 2017 and November 2019 at the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences, and Peking Union Medical College. Treatment efficacy was assessed by investigators by evaluating the objective response rate (ORR) and disease control rate (DCR). Overall survival (OS) was assessed using Cox proportional hazards models. The adverse events were also analyzed.
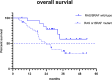
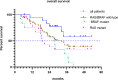
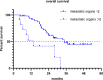
Results: Sixty-one consecutive patients were examined and followed up for survival. As of November 8, 2021, the median follow-up time was 35.4 months. Disease progression and death occurred in 50 (82%) and 38 (62%) patients, respectively. The median treatment duration of XELOXIRI with or without bevacizumab or cetuximab was 10 cycles (range, 1-12 cycles). The median OS and PFS were 32.2 months (95%CI [24.8-39.6]) and 9.3 months (95% CI [8.1-10.5]), respectively. The ORR of 48 patients with measurable lesions was 70.8%, and the DCR was 89.6%. RAS/BRAF wild-type (HR 0.39; 95% CI [0.16-0.96], p = 0.04) and metastatic organs > 2 (HR 3.25; 95% CI [1.34-7.87], p = 0.009) were independent prognostic factors for OS. The incidence of any grade of adverse events (AEs) was 96.7% (59/61). Grade ≥ 3 AEs included neutropenia (19.7%), leukopenia (9.8%), diarrhea (3.3%), vomiting (3.3%), febrile neutropenia (1.6%), and thrombocytopenia (1.6%). No treatment-related death occurred.
Conclusion: The use of the XELOXIRI regimen with or without a targeted drug was effective, with a manageable toxicity profile in Chinese patients with mCRC.
Keywords: Capecitabine; Colorectal cancer; Irinotecan; Oxaliplatin; Triplet chemotherapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as competing interests.
Figures
Similar articles
-
Efficacy and safety of triplet regimen capecitabine, oxaliplatin, and irinotecan (XELOXIRI) as first-line chemotherapy for advanced pancreatic cancer.BMC Cancer. 2025 Mar 12;25(1):449. doi: 10.1186/s12885-025-13799-5. BMC Cancer. 2025. PMID: 40075310 Free PMC article.
-
QUATTRO-II randomized trial: CAPOXIRI+bevacizumab vs. FOLFOXIRI+bevacizumab as first-line treatment in patients with mCRC.Med. 2024 Sep 13;5(9):1164-1177.e3. doi: 10.1016/j.medj.2024.05.012. Epub 2024 Jun 19. Med. 2024. PMID: 38901425 Clinical Trial.
-
Phase I/II trial of capecitabine, oxaliplatin, and irinotecan in combination with bevacizumab in first line treatment of metastatic colorectal cancer.Cancer Med. 2015 Oct;4(10):1505-13. doi: 10.1002/cam4.497. Epub 2015 Jul 24. Cancer Med. 2015. PMID: 26207614 Free PMC article. Clinical Trial.
-
Efficacy and safety of bevacizumab plus capecitabine and irinotecan regimen for metastatic colorectal cancer.Med Oncol. 2010 Sep;27(3):585-91. doi: 10.1007/s12032-009-9253-5. Epub 2009 Jun 13. Med Oncol. 2010. PMID: 19526201 Review.
-
Does the Chemotherapy Backbone Impact on the Efficacy of Targeted Agents in Metastatic Colorectal Cancer? A Systematic Review and Meta-Analysis of the Literature.PLoS One. 2015 Aug 14;10(8):e0135599. doi: 10.1371/journal.pone.0135599. eCollection 2015. PLoS One. 2015. PMID: 26275292 Free PMC article.
Cited by
-
Prognostic Nutritional Index is Related to All-Cause Mortality in Patients With Stage IV Colorectal Cancer Treated With Capecitabine: Single-Center 24-Month Observational Study in Vietnam.J Clin Lab Anal. 2024 Nov;38(21):e25112. doi: 10.1002/jcla.25112. Epub 2024 Oct 8. J Clin Lab Anal. 2024. PMID: 39380366 Free PMC article.
-
Targeting drug resistant colorectal cancer with apigenin nanoarchitectures.Transl Oncol. 2025 Sep;59:102455. doi: 10.1016/j.tranon.2025.102455. Epub 2025 Jun 24. Transl Oncol. 2025. PMID: 40561797 Free PMC article. Review.
-
Efficacy and safety of triplet regimen capecitabine, oxaliplatin, and irinotecan (XELOXIRI) as first-line chemotherapy for advanced pancreatic cancer.BMC Cancer. 2025 Mar 12;25(1):449. doi: 10.1186/s12885-025-13799-5. BMC Cancer. 2025. PMID: 40075310 Free PMC article.
-
Possible Involvement of Long Non-Coding RNAs GNAS-AS1 and MIR205HG in the Modulation of 5-Fluorouracil Chemosensitivity in Colon Cancer Cells through Increased Extracellular Release of Exosomes.Noncoding RNA. 2024 Apr 15;10(2):25. doi: 10.3390/ncrna10020025. Noncoding RNA. 2024. PMID: 38668383 Free PMC article.
-
Gastrointestinal Cancers with Consideration of DPD and UGT1A1 Plasma Levels: Chemotherapy-Related Toxicity.Life (Basel). 2025 Jul 4;15(7):1071. doi: 10.3390/life15071071. Life (Basel). 2025. PMID: 40724574 Free PMC article.
References
-
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/jco.2006.09.0928. - DOI - PubMed
-
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/s1470-2045(15)00122-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

