Automated diagnosis and prognosis of COVID-19 pneumonia from initial ER chest X-rays using deep learning
- PMID: 35864468
- PMCID: PMC9301895
- DOI: 10.1186/s12879-022-07617-7
Automated diagnosis and prognosis of COVID-19 pneumonia from initial ER chest X-rays using deep learning
Abstract
Background: Airspace disease as seen on chest X-rays is an important point in triage for patients initially presenting to the emergency department with suspected COVID-19 infection. The purpose of this study is to evaluate a previously trained interpretable deep learning algorithm for the diagnosis and prognosis of COVID-19 pneumonia from chest X-rays obtained in the ED.
Methods: This retrospective study included 2456 (50% RT-PCR positive for COVID-19) adult patients who received both a chest X-ray and SARS-CoV-2 RT-PCR test from January 2020 to March of 2021 in the emergency department at a single U.S.
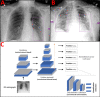
Institution: A total of 2000 patients were included as an additional training cohort and 456 patients in the randomized internal holdout testing cohort for a previously trained Siemens AI-Radiology Companion deep learning convolutional neural network algorithm. Three cardiothoracic fellowship-trained radiologists systematically evaluated each chest X-ray and generated an airspace disease area-based severity score which was compared against the same score produced by artificial intelligence. The interobserver agreement, diagnostic accuracy, and predictive capability for inpatient outcomes were assessed. Principal statistical tests used in this study include both univariate and multivariate logistic regression.
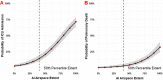
Results: Overall ICC was 0.820 (95% CI 0.790-0.840). The diagnostic AUC for SARS-CoV-2 RT-PCR positivity was 0.890 (95% CI 0.861-0.920) for the neural network and 0.936 (95% CI 0.918-0.960) for radiologists. Airspace opacities score by AI alone predicted ICU admission (AUC = 0.870) and mortality (0.829) in all patients. Addition of age and BMI into a multivariate log model improved mortality prediction (AUC = 0.906).
Conclusion: The deep learning algorithm provides an accurate and interpretable assessment of the disease burden in COVID-19 pneumonia on chest radiographs. The reported severity scores correlate with expert assessment and accurately predicts important clinical outcomes. The algorithm contributes additional prognostic information not currently incorporated into patient management.
Keywords: COVID-19; Critical care; Deep learning; Pulmonology; Radiology.
© 2022. The Author(s).
Conflict of interest statement
Florin Ghesu PhD, Awais Mansoor PhD, Philipp Hoelzer PhD, Mathis Zimmermann MS MBA are employees of Seimens Healthineers. Funding: Jonathan Sperl PhD receives research funding from Siemens Healthineers. Jeremy R. Burt MD has an ownership interest in YellowDot Innovations, is a medical consultant for Canatu, and receives research funding from Siemens Healthineers. The remaining authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Prognostication of patients with COVID-19 using artificial intelligence based on chest x-rays and clinical data: a retrospective study.Lancet Digit Health. 2021 May;3(5):e286-e294. doi: 10.1016/S2589-7500(21)00039-X. Epub 2021 Mar 24. Lancet Digit Health. 2021. PMID: 33773969 Free PMC article.
-
An Interpretable Chest CT Deep Learning Algorithm for Quantification of COVID-19 Lung Disease and Prediction of Inpatient Morbidity and Mortality.Acad Radiol. 2022 Aug;29(8):1178-1188. doi: 10.1016/j.acra.2022.03.023. Epub 2022 Apr 4. Acad Radiol. 2022. PMID: 35610114 Free PMC article.
-
Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in COVID-19 patients: analysis of 697 Italian patients.Eur Radiol. 2021 Mar;31(3):1770-1779. doi: 10.1007/s00330-020-07269-8. Epub 2020 Sep 18. Eur Radiol. 2021. PMID: 32945968 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
Cited by
-
Deep Learning With Chest Radiographs for Making Prognoses in Patients With COVID-19: Retrospective Cohort Study.J Med Internet Res. 2023 Feb 16;25:e42717. doi: 10.2196/42717. J Med Internet Res. 2023. PMID: 36795468 Free PMC article.
-
Artificial intelligence-based model for COVID-19 prognosis incorporating chest radiographs and clinical data; a retrospective model development and validation study.Br J Radiol. 2022 Dec 1;95(1140):20220058. doi: 10.1259/bjr.20220058. Epub 2022 Oct 27. Br J Radiol. 2022. PMID: 36193755 Free PMC article. Clinical Trial.
-
Comprehensive analysis of clinical data for COVID-19 outcome estimation with machine learning models.Biomed Signal Process Control. 2023 Jul;84:104818. doi: 10.1016/j.bspc.2023.104818. Epub 2023 Mar 9. Biomed Signal Process Control. 2023. PMID: 36915863 Free PMC article.
-
Artificial Intelligence in Optimizing the Functioning of Emergency Departments; a Systematic Review of Current Solutions.Arch Acad Emerg Med. 2024 Jan 27;12(1):e22. doi: 10.22037/aaem.v12i1.2110. eCollection 2024. Arch Acad Emerg Med. 2024. PMID: 38572221 Free PMC article. Review.
-
Artificial Intelligence-Empowered Radiology-Current Status and Critical Review.Diagnostics (Basel). 2025 Jan 24;15(3):282. doi: 10.3390/diagnostics15030282. Diagnostics (Basel). 2025. PMID: 39941212 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

