Beneficial and pathogenic plant-microbe interactions during flooding stress
- PMID: 35864739
- PMCID: PMC9543564
- DOI: 10.1111/pce.14403
Beneficial and pathogenic plant-microbe interactions during flooding stress
Abstract
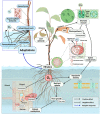
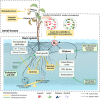
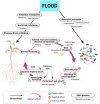
The number and intensity of flood events will likely increase in the future, raising the risk of flooding stress in terrestrial plants. Understanding flood effects on plant physiology and plant-associated microbes is key to alleviate flooding stress in sensitive species and ecosystems. Reduced oxygen supply is the main constrain to the plant and its associated microbiome. Hypoxic conditions hamper root aerobic respiration and, consequently, hydraulic conductance, nutrient uptake, and plant growth and development. Hypoxia favours the presence of anaerobic microbes in the rhizosphere and roots with potential negative effects to the plant due to their pathogenic behaviour or their soil denitrification ability. Moreover, plant physiological and metabolic changes induced by flooding stress may also cause dysbiotic changes in endosphere and rhizosphere microbial composition. The negative effects of flooding stress on the holobiont (i.e., the host plant and its associated microbiome) can be mitigated once the plant displays adaptive responses to increase oxygen uptake. Stress relief could also arise from the positive effect of certain beneficial microbes, such as mycorrhiza or dark septate endophytes. More research is needed to explore the spiralling, feedback flood responses of plant and microbes if we want to promote plant flood tolerance from a holobiont perspective.
Keywords: flood resilience; holobiont; inundation; pathogens; phyllosphere; plant endophytes; rhizosphere; waterlogging.
© 2022 The Authors. Plant, Cell & Environment published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Beneficial Root-Associated Microbiome during Drought and Flooding Stress in Plants.Pak J Biol Sci. 2023 Apr;26(5):287-299. doi: 10.3923/pjbs.2023.287.299. Pak J Biol Sci. 2023. PMID: 37859559 Review.
-
Flooding episodes and seed treatment influence the microbiome diversity and function in the soybean root and rhizosphere.Sci Total Environ. 2025 Jun 20;982:179554. doi: 10.1016/j.scitotenv.2025.179554. Epub 2025 May 13. Sci Total Environ. 2025. PMID: 40367854
-
Beneficial microbes going underground of root immunity.Plant Cell Environ. 2019 Oct;42(10):2860-2870. doi: 10.1111/pce.13632. Epub 2019 Aug 5. Plant Cell Environ. 2019. PMID: 31353481 Free PMC article. Review.
-
Expression of root-related transcription factors associated with flooding tolerance of soybean (Glycine max).Int J Mol Sci. 2014 Sep 29;15(10):17622-43. doi: 10.3390/ijms151017622. Int J Mol Sci. 2014. PMID: 25268626 Free PMC article.
-
Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture.Biotechnol Adv. 2016 Nov 15;34(7):1245-1259. doi: 10.1016/j.biotechadv.2016.08.005. Epub 2016 Aug 30. Biotechnol Adv. 2016. PMID: 27587331 Review.
Cited by
-
Soil microbiome analysis reveals effects of periodic waterlogging stress on sugarcane growth.PLoS One. 2023 Nov 2;18(11):e0293834. doi: 10.1371/journal.pone.0293834. eCollection 2023. PLoS One. 2023. PMID: 37917788 Free PMC article.
-
The adaptive microbiome hypothesis and immune interactions in amphibian mucus.Dev Comp Immunol. 2023 Aug;145:104690. doi: 10.1016/j.dci.2023.104690. Epub 2023 Mar 29. Dev Comp Immunol. 2023. PMID: 37001710 Free PMC article. Review.
-
Looking for Resistance to Soft Rot Disease of Potatoes Facing Environmental Hypoxia.Int J Mol Sci. 2024 Mar 28;25(7):3757. doi: 10.3390/ijms25073757. Int J Mol Sci. 2024. PMID: 38612570 Free PMC article. Review.
-
Warming Scenarios and Phytophthora cinnamomi Infection in Chestnut (Castanea sativa Mill.).Plants (Basel). 2023 Jan 26;12(3):556. doi: 10.3390/plants12030556. Plants (Basel). 2023. PMID: 36771639 Free PMC article.
-
Plant Beneficial Bacteria and Their Potential Applications in Vertical Farming Systems.Plants (Basel). 2023 Jan 15;12(2):400. doi: 10.3390/plants12020400. Plants (Basel). 2023. PMID: 36679113 Free PMC article. Review.
References
-
- Abanda‐Nkpwatt, D. , Musch, M. , Tschiersch, J. , Boettner, M. & Schwab, W. (2006) Molecular interaction between Methylobacterium extorquens and seedlings: Growth promotion, methanol consumption, and localization of the methanol emission site. Journal of Experimental Botany, 57, 4025–4032. - PubMed
-
- Armstrong, J. & Armstrong, W. (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany, 88, 1359–1370. - PubMed
-
- Baar, L. , Bastiaans, T. , Coevering, M.V & R., J. (2002) Ectomycorrhizal root development in wet Alder carr forests in response to desiccation and eutrophication. Mycorrhiza, 12, 147–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

