The Effects of Poria cocos on Rho Signaling-Induced Regulation of Mobility and F-Actin Aggregation in MK-801-Treated B35 and C6 Cells
- PMID: 35864844
- PMCID: PMC9296330
- DOI: 10.1155/2022/8225499
The Effects of Poria cocos on Rho Signaling-Induced Regulation of Mobility and F-Actin Aggregation in MK-801-Treated B35 and C6 Cells
Abstract
Methods: B35 neuronal cells and C6 glial cells were incubated with MK-801 for 7 days followed by MK-801, MK801 in combination with water extracts of P. cocos (PRP for P. cocos cum Radix Pini or WP for White Poria) treatment for an additional 7 days. Analysis of cell mobility, F-actin aggregation, and Rho signaling modulation was performed to clarify the roles of PRP or WP in MK-801-treated B35 and C6 cells.
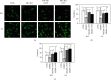
Results: MK-801 decreases B35 cell mobility, whereas the inhibited cell migration ability and F-actin aggregation in MK-801-treated B35 or C6 cells could be reversed by PRP or WP. The CDC42 expression in B35 or C6 cells would be reduced by MK-801 and restored by treating with PRP or WP. The RhoA expression was increased by MK-801 in both B35 and C6 cells but was differentially regulated by PRP or WP. In B35 cells, downregulation of PFN1, N-WASP, PAK1, and ARP2/3 induced by MK-801 can be reversely modulated by PRP or WP. PRP or WP reduced the increase in the p-MLC2 expression in B35 cells treated with MK-801. The reduction in ROCK1, PFN1, p-MLC2, and ARP2/3 expression in C6 cells induced by MK-801 was restored by PRP or WP. Reduced N-WASP and PAK1 expression was differentially regulated by PRP or WP in MK-801-treated C6 cells.
Copyright © 2022 Yi-Chyan Chen et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

