Novel insights into m6A modification of coding and non-coding RNAs in tumor biology: From molecular mechanisms to therapeutic significance
- PMID: 35864970
- PMCID: PMC9295064
- DOI: 10.7150/ijbs.73093
Novel insights into m6A modification of coding and non-coding RNAs in tumor biology: From molecular mechanisms to therapeutic significance
Abstract
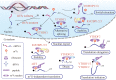
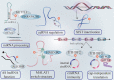
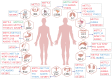
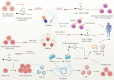
Accumulating evidence has revealed that m6A modification, the predominant RNA modification in eukaryotes, adds a novel layer of regulation to the gene expression. Dynamic and reversible m6A modification implements sophisticated and crucial functions in RNA metabolism, including generation, splicing, stability, and translation in messenger RNAs (mRNAs) and non-coding RNAs (ncRNAs). Furthermore, m6A modification plays a determining role in producing various m6A-labeling RNA outcomes, thereby affecting several functional processes, including tumorigenesis and progression. Herein, we highlighted current advances in m6A modification and the regulatory mechanisms underlying mRNAs and ncRNAs in distinct cancer stages. Meanwhile, we also focused on the therapeutic significance of m6A regulators in clinical cancer treatment.
Keywords: m6A; non-coding RNA; therapeutic target; tumor microenvironment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat Rev Genet. 2014;15:293–306. - PubMed
-
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975;4:379–86. - PubMed
-
- Rottman F, Shatkin AJ, Perry RP. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974;3:197–9. - PubMed
-
- Fustin J-M, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M. et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

