An Update on the Multifaceted Role of NF-kappaB in Endometriosis
- PMID: 35864971
- PMCID: PMC9295070
- DOI: 10.7150/ijbs.72707
An Update on the Multifaceted Role of NF-kappaB in Endometriosis
Abstract
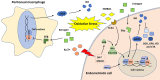
Endometriosis remains a common but challenging gynecological disease among reproductive-aged women with an unclear pathogenesis and limited therapeutic options. Numerous pieces of evidence suggest that NF-κB signaling, a major regulator of inflammatory responses, is overactive in endometriotic lesions and contributes to the onset, progression, and recurrence of endometriosis. Several factors, such as estrogen, progesterone, oxidative stress, and noncoding RNAs, can regulate NF-κB signaling in endometriosis. In the present review, we discuss the mechanisms by which these factors regulate NF-κB during endometriosis progression and provide an update on the role of NF-κB in affecting endometriotic cells, peritoneal macrophages (PMs) as well as endometriosis-related symptoms, such as pain and infertility. Furthermore, the preclinical drugs for blocking NF-κB signaling in endometriosis are summarized, including plant-derived medicines, NF-κB inhibitors, other known drugs, and the potential anti-NF-κB drugs predicted through the Drug-Gene Interaction Database. The present review discusses most of the studies concerning the multifaceted role of NF-κB signaling in endometriosis and provides a summary of NF-κB-targeted treatment in detail.
Keywords: NF-κB; endometriosis; peritoneal macrophage.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561–72. - PubMed
-
- Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nature reviews Endocrinology. 2014;10:261–75. - PubMed
-
- Jiang L, Yan Y, Liu Z, Wang Y. Inflammation and endometriosis. Frontiers in bioscience (Landmark edition) 2016;21:941–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

