Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation
- PMID: 35864981
- PMCID: PMC9294323
- DOI: 10.3389/fnins.2022.841036
Ceftriaxone as a Novel Therapeutic Agent for Hyperglutamatergic States: Bridging the Gap Between Preclinical Results and Clinical Translation
Abstract
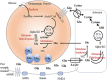
Dysregulation of glutamate homeostasis is a well-established core feature of neuropsychiatric disorders. Extracellular glutamate concentration is regulated by glutamate transporter 1 (GLT-1). The discovery of a beta-lactam antibiotic, ceftriaxone (CEF), as a safe compound with unique ability to upregulate GLT-1 sparked the interest in testing its efficacy as a novel therapeutic agent in animal models of neuropsychiatric disorders with hyperglutamatergic states. Indeed, more than 100 preclinical studies have shown the efficacy of CEF in attenuating the behavioral manifestations of various hyperglutamatergic brain disorders such as ischemic stroke, amyotrophic lateral sclerosis (ALS), seizure, Huntington's disease, and various aspects of drug use disorders. However, despite rich and promising preclinical data, only one large-scale clinical trial testing the efficacy of CEF in patients with ALS is reported. Unfortunately, in that study, there was no significant difference in survival between placebo- and CEF-treated patients. In this review, we discussed the translational potential of preclinical efficacy of CEF based on four different parameters: (1) initiation of CEF treatment in relation to induction of the hyperglutamatergic state, (2) onset of response in preclinical models in relation to onset of GLT-1 upregulation, (3) mechanisms of action of CEF on GLT-1 expression and function, and (4) non-GLT-1-mediated mechanisms for CEF. Our detailed review of the literature brings new insights into underlying molecular mechanisms correlating the preclinical efficacy of CEF. We concluded here that CEF may be clinically effective in selected cases in acute and transient hyperglutamatergic states such as early drug withdrawal conditions.
Keywords: GLT-1; ceftriaxone; glutamate; neurological disorder; psychiatric disorder.
Copyright © 2022 Abulseoud, Alasmari, Hussein and Sari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aal-Aaboda M., Alhaddad H., Osowik F., Nauli S. M., Sari Y. (2015). Effects of (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J. Neurosci. Res. 93 930–937. 10.1002/jnr.23554 - DOI - PMC - PubMed
-
- Abulseoud O. A., Miller J. D., Wu J., Choi D. S., Holschneider D. P. (2012). Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 1456 14–21. 10.1016/j.brainres.2012.03.045 - DOI - PMC - PubMed
-
- Abulseoud O. A., Ross T. J., Nam H. W., Caparelli E. C., Tennekoon M., Schleyer B., et al. (2020). Short-term nicotine deprivation alters dorsal anterior cingulate glutamate concentration and concomitant cingulate-cortical functional connectivity. Neuropsychopharmacology 45 1920–1930. 10.1038/s41386-020-0741-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

