Beyond LIF Neurons on Neuromorphic Hardware
- PMID: 35864984
- PMCID: PMC9294628
- DOI: 10.3389/fnins.2022.881598
Beyond LIF Neurons on Neuromorphic Hardware
Abstract
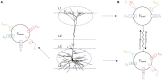
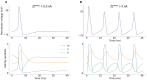
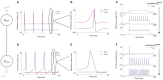
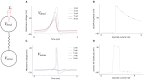
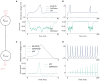
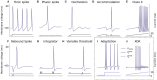
Neuromorphic systems aim to provide accelerated low-power simulation of Spiking Neural Networks (SNNs), typically featuring simple and efficient neuron models such as the Leaky Integrate-and-Fire (LIF) model. Biologically plausible neuron models developed by neuroscientists are largely ignored in neuromorphic computing due to their increased computational costs. This work bridges this gap through implementation and evaluation of a single compartment Hodgkin-Huxley (HH) neuron and a multi-compartment neuron incorporating dendritic computation on the SpiNNaker, and SpiNNaker2 prototype neuromorphic systems. Numerical accuracy of the model implementations is benchmarked against reference models in the NEURON simulation environment, with excellent agreement achieved by both the fixed- and floating-point SpiNNaker implementations. The computational cost is evaluated in terms of timing measurements profiling neural state updates. While the additional model complexity understandably increases computation times relative to LIF models, it was found a wallclock time increase of only 8× was observed for the HH neuron (11× for the mutlicompartment model), demonstrating the potential of hardware accelerators in the next-generation neuromorphic system to optimize implementation of complex neuron models. The benefits of models directly corresponding to biophysiological data are demonstrated: HH neurons are able to express a range of output behaviors not captured by LIF neurons; and the dendritic compartment provides the first implementation of a spiking multi-compartment neuron model with XOR-solving capabilities on neuromorphic hardware. The work paves the way for inclusion of more biologically representative neuron models in neuromorphic systems, and showcases the benefits of hardware accelerators included in the next-generation SpiNNaker2 architecture.
Keywords: Hodgkin-Huxley; SpiNNaker; dendritic computation; neuromorphic computing; neuronal modeling; spiking neural networks.
Copyright © 2022 Ward and Rhodes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Benjamin B. V., Gao P., McQuinn E., Choudhary S., Chandrasekaran A. R., Bussat J., et al. (2014). Neurogrid: a mixed-analog-digital multichip system for large-scale neural simulations. Proc. IEEE 102, 699–716. 10.1109/JPROC.2014.2313565 - DOI
-
- Cyr A., Thériault F., Chartier S. (2020). Revisiting the XOR problem: a neurorobotic implementation. Neural Comput. Appl. 32, 9965–9973. 10.1007/s00521-019-04522-0 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

