Target-based anticancer indole derivatives and insight into structure‒activity relationship: A mechanistic review update (2018-2021)
- PMID: 35865090
- PMCID: PMC9293743
- DOI: 10.1016/j.apsb.2022.03.021
Target-based anticancer indole derivatives and insight into structure‒activity relationship: A mechanistic review update (2018-2021)
Abstract
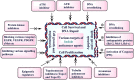
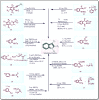
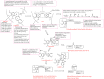
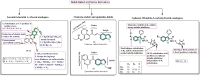
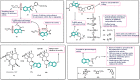
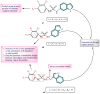
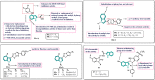
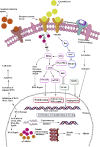
Cancer, which is the uncontrolled growth of cells, is the second leading cause of death after heart disease. Targeting drugs, especially to specific genes and proteins involved in growth and survival of cancer cells, is the prime need of research world-wide. Indole moiety, which is a combination of aromatic-heterocyclic compounds, is a constructive scaffold for the development of novel leads. Owing to its bioavailability, high unique chemical properties and significant pharmacological behaviours, indole is considered as the most inquisitive scaffold for anticancer drug research. This is illustrated by the fact that the U.S. Food and Drug Administration (FDA) has recently approved several indole-based anticancer agents such as panobinostat, alectinib, sunitinib, osimertinib, anlotinib and nintedanib for clinical use. Furthermore, hundreds of studies on the synthesis and activity of the indole ring have been published in the last three years. Taking into account the facts stated above, we have presented the most recent advances in medicinal chemistry of indole derivatives, encompassing hot articles published between 2018 and 2021 in anticancer drug research. The recent advances made towards the synthesis of promising indole-based anticancer compounds that may act via various targets such as topoisomerase, tubulin, apoptosis, aromatase, kinases, etc., have been discussed. This review also summarizes some of the recent efficient green chemical synthesis for indole rings using various catalysts for the period during 2018-2021. The review also covers the synthesis, structure‒activity relationship, and mechanism by which these leads have demonstrated improved and promising anticancer activity. Indole molecules under clinical and preclinical stages are classified into groups based on their cancer targets and presented in tabular form, along with their mechanism of action. The goal of this review article is to point the way for medicinal chemists to design and develop effective indole-based anticancer agents.
Keywords: Anticancer; Apoptosis; Aromatase inhibitors; Indole; Structure‒activity relationship; Synthesis; Topoisomerase; Tubulin inhibitors.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
Indole Antitumor Agents in Nanotechnology Formulations: An Overview.Pharmaceutics. 2023 Jun 25;15(7):1815. doi: 10.3390/pharmaceutics15071815. Pharmaceutics. 2023. PMID: 37514002 Free PMC article. Review.
-
Recent Development in Indole Derivatives as Anticancer Agents for Breast Cancer.Anticancer Agents Med Chem. 2019;19(8):962-983. doi: 10.2174/1871520619666190312125602. Anticancer Agents Med Chem. 2019. PMID: 30864529 Review.
-
Synthesis and Preclinical Evaluation of Indole Triazole Conjugates as Microtubule Targeting Agents that are Effective against MCF-7 Breast Cancer Cell Lines.Anticancer Agents Med Chem. 2021;21(8):1047-1055. doi: 10.2174/1871520620666200925102940. Anticancer Agents Med Chem. 2021. PMID: 32981511
-
Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach.Anticancer Agents Med Chem. 2021;21(14):1802-1824. doi: 10.2174/1871520621999210104192644. Anticancer Agents Med Chem. 2021. PMID: 33397272 Review.
-
Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives.Bioorg Chem. 2019 Aug;89:103021. doi: 10.1016/j.bioorg.2019.103021. Epub 2019 May 30. Bioorg Chem. 2019. PMID: 31176854 Review.
Cited by
-
Research status of indole-modified natural products.RSC Med Chem. 2023 Oct 17;14(12):2535-2563. doi: 10.1039/d3md00560g. eCollection 2023 Dec 13. RSC Med Chem. 2023. PMID: 38107170 Free PMC article. Review.
-
Metformin Preserves Insulin Secretion in Pancreatic β-cells through FGF21/Akt Pathway In vitro and In vivo.Comb Chem High Throughput Screen. 2024;27(18):2691-2698. doi: 10.2174/0113862073246747230920170201. Comb Chem High Throughput Screen. 2024. PMID: 37855357
-
Platinum(IV) Prodrugs Incorporating an Indole-Based Derivative, 5-Benzyloxyindole-3-Acetic Acid in the Axial Position Exhibit Prominent Anticancer Activity.Int J Mol Sci. 2024 Feb 11;25(4):2181. doi: 10.3390/ijms25042181. Int J Mol Sci. 2024. PMID: 38396859 Free PMC article.
-
Thiazole conjugated amino acid derivatives as potent cytotoxic agents.RSC Adv. 2025 Jul 28;15(33):26860-26872. doi: 10.1039/d5ra04425a. eCollection 2025 Jul 25. RSC Adv. 2025. PMID: 40727284 Free PMC article.
-
Recent Development in the Search for Epidermal Growth Factor Receptor (EGFR) Inhibitors based on the Indole Pharmacophore.Curr Top Med Chem. 2024;24(7):581-613. doi: 10.2174/0115680266264206231020111820. Curr Top Med Chem. 2024. PMID: 37909440 Review.
References
-
- Ali I., Lone M.N., Al-Othman Z.A., Al-Warthan A., Sanagi M.M. Heterocyclic scaffolds: centrality in anticancer drug development. Curr Drug Targets. 2015;16:711–734. - PubMed
-
- Gholap S.S. Pyrrole: an emerging scaffold for construction of valuable therapeutic agents. Eur J Med Chem. 2016;110:13–31. - PubMed
-
- Ahmad S., Alam O., Naim M.J., Shaquiquzzaman M., MumtajAlam M., Iqbal M. Pyrrole: an insight into recent pharmacological advances with structure activity relationship. Eur J Med Chem. 2018;157:527–561. - PubMed
-
- Tantawy M.A., Nafie M.S., Elmegeed G.A., Ali I.A.I. Auspicious role of the steroidal heterocyclic derivatives as a platform for anti-cancer drugs. Bioorg Chem. 2017;73:128–146. - PubMed
-
- Sharma V., Kumar P., Pathak D. Biological importance of the indole nucleus in recent years: a comprehensive review. J Heterocycl Chem. 2010;47:491–502.
Publication types
LinkOut - more resources
Full Text Sources

