Generation of α Gal-enhanced bifunctional tumor vaccine
- PMID: 35865091
- PMCID: PMC9293690
- DOI: 10.1016/j.apsb.2022.03.002
Generation of α Gal-enhanced bifunctional tumor vaccine
Erratum in
-
Erratum: Author correction to "Generation of αGal-enhanced bifunctional tumor vaccine" [Acta Pharm Sin B 12 (2022) 3177-3186].Acta Pharm Sin B. 2025 Feb;15(2):1207. doi: 10.1016/j.apsb.2024.11.021. Epub 2024 Nov 29. Acta Pharm Sin B. 2025. PMID: 40177562 Free PMC article.
Abstract
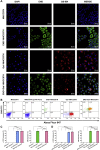
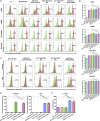
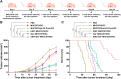
Hepatocellular carcinoma (HCC) is a common malignant tumor with poor prognosis and high mortality. In this study, we demonstrated a novel vaccine targeting HCC and tumor neovascular endothelial cells by fusing recombinant MHCC97H cells expressing porcine α-1,3-galactose epitopes (αGal) and endorphin extracellular domains (END) with dendritic cells (DCs) from healthy volunteers. END+/Gal+-MHCC97H/DC fusion cells induced cytotoxic T lymphocytes (CTLs) and secretion of interferon-gamma (IFN-γ). CTLs targeted cells expressing αGal and END and tumor angiogenesis. The fused cell vaccine can effectively inhibit tumor growth and prolong the survival time of human hepatoma mice, indicating the high clinical potential of this new cell based vaccine.
Keywords: Cytotoxic T lymphocytes; Dendritic cells; Endoglin; Fusion cells; Hepatocellular carcinoma; Immunotherapy; Tumor neovascular endothelial cells; αGal.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures



Similar articles
-
Novel fusion cells derived from tumor cells expressing the heterologous α-galactose epitope and dendritic cells effectively target cancer.Vaccine. 2019 Feb 8;37(7):926-936. doi: 10.1016/j.vaccine.2019.01.004. Epub 2019 Jan 17. Vaccine. 2019. PMID: 30661833
-
Dendritic cells-mediated CTLs targeting hepatocellular carcinoma stem cells.Cancer Biol Ther. 2010 Aug 15;10(4):368-75. doi: 10.4161/cbt.10.4.12440. Epub 2010 Aug 22. Cancer Biol Ther. 2010. PMID: 20581468
-
[Inducing hepatocellular carcinoma-specific cytotoxic T lymphocyte response using formed by fusion of FastDCs and allogeneic human hepatocellular carcinoma cells].Zhonghua Yi Xue Za Zhi. 2005 Dec 14;85(47):3332-6. Zhonghua Yi Xue Za Zhi. 2005. PMID: 16409838 Chinese.
-
Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models.J Hepatol. 2017 Oct;67(4):739-748. doi: 10.1016/j.jhep.2017.05.019. Epub 2017 May 24. J Hepatol. 2017. PMID: 28549917
-
Induction of cytotoxic T lymphocytes against human cancer cell lines using dendritic cell-tumor cell hybrids generated by a newly developed electrofusion technique.Int J Oncol. 2006 Sep;29(3):531-9. Int J Oncol. 2006. PMID: 16865268
Cited by
-
Harnessing Dendritic Cell Function in Hepatocellular Carcinoma: Advances in Immunotherapy and Therapeutic Strategies.Vaccines (Basel). 2025 May 4;13(5):496. doi: 10.3390/vaccines13050496. Vaccines (Basel). 2025. PMID: 40432108 Free PMC article. Review.
-
A metabolic intervention strategy to break evolutionary adaptability of tumor for reinforced immunotherapy.Acta Pharm Sin B. 2023 Feb;13(2):775-786. doi: 10.1016/j.apsb.2022.10.021. Epub 2022 Oct 28. Acta Pharm Sin B. 2023. PMID: 36873182 Free PMC article.
References
-
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Rimassa L., Personeni N., Czauderna C., Foerster F., Galle P. Systemic treatment of HCC in special populations. J Hepatol. 2021;74:931–943. - PubMed
-
- Vinanica N., Yong A., Wong D., Png Y.T., Seow S.V., Imamura M., et al. Specific stimulation of T lymphocytes with erythropoietin for adoptive immunotherapy. Blood. 2020;135:668–679. - PubMed
LinkOut - more resources
Full Text Sources

