Density functional theory analysis for H2S adsorption on pyridinic N- and oxidized N-doped graphenes
- PMID: 35865209
- PMCID: PMC9264117
- DOI: 10.1039/d2ra00898j
Density functional theory analysis for H2S adsorption on pyridinic N- and oxidized N-doped graphenes
Abstract
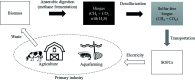
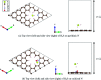
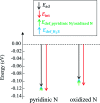
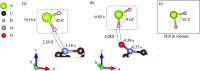
Biomass discharged from primary industries can be converted into methane by fermentation. This methane is used for generating electricity with solid oxide fuel cells (SOFCs). This methane fermentation provides H2S, which reduces the efficiency of SOFCs even at a level as low as a few parts per million. It has been experimentally reported that a nitrogen (N)-doped graphene-based material known as pyridinic N removes H2S via an oxidation reaction compared with another graphene-based material known as oxidized N. To understand this experimental result, we investigated H2S adsorption on pyridinic N and oxidized N by a density functional theory analysis and further examined the activation barrier of dissociation reactions. We found that the adsorption of H2S on pyridinic N is more stable than that on oxidized N. In addition, the H2S dissociation reaction occurs only on pyridinic N.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Pyridinic-Type N-Doped Graphene on Cobalt Substrate as Efficient Electrocatalyst for Oxygen Reduction Reaction in Acidic Solution in Fuel Cell.J Phys Chem Lett. 2021 Apr 15;12(14):3552-3559. doi: 10.1021/acs.jpclett.1c00198. Epub 2021 Apr 5. J Phys Chem Lett. 2021. PMID: 33819038
-
Improved performance of graphene doped with pyridinic N for Li-ion battery: a density functional theory model.Phys Chem Chem Phys. 2013 Aug 21;15(31):12982-7. doi: 10.1039/c3cp51987b. Phys Chem Chem Phys. 2013. PMID: 23817454
-
Microscopic effects of the bonding configuration of nitrogen-doped graphene on its reactivity toward hydrogen peroxide reduction reaction.Phys Chem Chem Phys. 2013 May 14;15(18):6920-8. doi: 10.1039/c3cp50900a. Epub 2013 Apr 3. Phys Chem Chem Phys. 2013. PMID: 23549636
-
Boron and pyridinic nitrogen-doped graphene as potential catalysts for rechargeable non-aqueous sodium-air batteries.RSC Adv. 2020 Jun 9;10(36):21387-21398. doi: 10.1039/d0ra03126g. eCollection 2020 Jun 2. RSC Adv. 2020. PMID: 35518781 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
References
-
- U. S. Energy Information Administration (EIA), April2021 Monthly Energy Review, DOE/EIA-0035(2021/12), Washington, DC, December 22, 2021, https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf
-
- Mercure J. F. Pollitt H. Viñuales J. E. Edwards N. R. Holden P. B. Chewpreecha U. Salas P. Sognnaes I. Lam A. Knobloch F. Nat. Clim. Change. 2018;8:588–593.
-
- Looney B., BP Statistical Review of World Energy, 2020, p. 66
-
- Mohr S. H. Wang J. Ellem G. Ward J. Giurco D. Fuel. 2015;141:120–135.
-
- López González E. Isorna Llerena F. Silva Pérez M. Rosa Iglesias F. Guerra Macho J. Int. J. Hydrogen Energy. 2015;40:5518–5525.
LinkOut - more resources
Full Text Sources

