Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases
- PMID: 35865314
- PMCID: PMC9294175
- DOI: 10.3389/fendo.2022.908868
Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases
Abstract
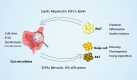
The rapid increase of obesity and associated diseases has become a major global health problem. Adipose tissues are critical for whole-body homeostasis. The gut microbiota has been recognized as a significant environmental factor in the maintenance of energy homeostasis and host immunity. A growing body of evidence suggests that the gut microbiota regulates host metabolism through a close cross-talk with adipose tissues. It modulates energy expenditure and alleviates obesity by promoting energy expenditure, but it also produces specific metabolites and structural components that may act as the central factors in the pathogenesis of inflammation, insulin resistance, and obesity. Understanding the relationship between gut microbiota and adipose tissues may provide potential intervention strategies to treat obesity and associated diseases. In this review, we focus on recent advances in the gut microbiota and its actions on adipose tissues and highlight the joint actions of the gut microbiota and adipose tissue with each other in the regulation of energy metabolism.
Keywords: adipose tissues; energy metabolism; gut microbiota; inflammation; obesity.
Copyright © 2022 Wu, Wang, Xie and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Yupanqui-Lozno H, Bastarrachea RA, Yupanqui-Velazco ME, Alvarez-Jaramillo M, Medina-Méndez E, Giraldo-Peña AP, et al. . Congenital Leptin Deficiency and Leptin Gene Missense Mutation Found in Two Colombian Sisters With Severe Obesity. Genes (Basel) (2019) 10(5):342. doi: 10.3390/genes10050342 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

