Efficacy and Safety of Qinpi Tongfeng Formula in the Treatment of Acute Gouty Arthritis: A Double-Blind, Double-Dummy, Multicenter, Randomized Controlled Trial
- PMID: 35865342
- PMCID: PMC9296295
- DOI: 10.1155/2022/7873426
Efficacy and Safety of Qinpi Tongfeng Formula in the Treatment of Acute Gouty Arthritis: A Double-Blind, Double-Dummy, Multicenter, Randomized Controlled Trial
Abstract
Objective: Traditional Chinese medicine (TCM) has certain curative effect against acute gouty arthritis (AGA), but it lacks high-quality evidence-based studies. In this randomized controlled trial, we try to evaluate the clinical efficacy and safety of Qinpi Tongfeng Formula (QPTFF) in the treatment of AGA.
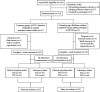
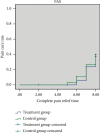
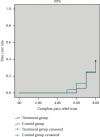
Methods: One hundred and fourteen patients with AGA (damp heat accumulation syndrome) who met the inclusion and exclusion criteria were randomly divided into treatment group and control group in a ratio of 1 : 1. Patients in the treatment group were treated with QPTFF, and patients in the control group were treated with diclofenac sodium sustained-release tablets for 7 days. The primary outcome measure was the change in visual analog scale (VAS) score for pain from the baseline to day 8. The secondary outcome measures were joint symptom score, TCM syndrome score, total effective rate, pain cure rate, complete pain relief time, patient satisfaction score, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum uric acid level. The safety outcome measures were routine blood test, urinalysis, liver function including alanine aminotransferase and aspartate aminotransferase, renal function including blood urea nitrogen and serum creatinine, and the rate of treatment-related adverse events (TRAEs).
Results: 105 patients with 53 in the treatment group and 52 in the control group completed the 7-day treatment. There was no significant difference between two groups in demographic characteristics, VAS score for pain, joint symptom score, TCM syndrome score, ESR, CRP, and serum uric acid level before enrollment at baseline (based on both the full analysis set (FAS) and per protocol set (PPS), P > 0.05). The 95% confidence interval of the difference between the eighth and first VAS score for pain of the two groups was (-0.57, 0.42) in FAS and (-0.48, 0.47) in PPS. The lower bound of both FAS and PPS is greater than the bound value of -0.7. On day 8, there was no significant difference between the two groups in joint symptom score, TCM syndrome score, total effective rate, pain cure rate, complete pain relief time, patient satisfaction score, ESR, and CRP (FAS and PPS, P > 0.05). The serum uric acid level and TRAEs in the treatment group were significantly lower than those in the control group (FAS and PPS, P < 0.05).
Conclusions: QPTFF could alleviate the symptoms of patients with AGA, which is not inferior to diclofenac sodium sustained-release tablets in analgesic. Moreover, QPTFF overmatches diclofenac sodium sustained-release tablets in decreasing serum uric acid level and TRAEs. Therefore, the results provide reliable foundation for QPTTF in the treatment of AGA. Trial Registration. This study protocol was registered in Chinese Clinical Trial Registry (registration number: ChiCTR2100050638).
Copyright © 2022 Yihua Fan et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Johannsdottir G. A., Palsson O., Jonsson H., Gudbjornsson B. Gout—a treatable condition. Laeknabladid . 2008;104(4):177–186. - PubMed
-
- Kaly L., Bilder I., Rozenbaum M., et al. Acute gout sacroiliitis. The Israel Medical Association Journal: The Israel Medical Association Journal . 2021;23(3):191–192. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

